
We recently recalled in these pages the discovery of ionising radiation by Roentgen, the fate of atomic bomb survivors of Hiroshima and Nagasaki and how – 75 years later – we still use their outcome data to estimate harms from radiation exposure, and the impacts of both on modern interventional cardiology1. It is only through the leveraging of the benefits of ionising radiation technology that the success of interventional cardiology has been possible. The detrimental effects of radiation exposure for both patients and healthcare workers – both tissue effects and stochastic (probabilistic) effects – are the price that must be paid for its use.
At the patient level, cardiology procedures represent an important source of medical radiation2. One survey in 2014 showed that interventional cardiology contributed about 1/5 of the medical radiation doses received by patients despite being derived from less than 1% of the medical radiation procedures performed3. Entrance air kerma and kerma-area product (also known as dose-area product) are the most meaningful measurement parameters for patient exposure, much better than total fluoroscopic time for example, which does not account for a variety of parameters including patient-related factors and technical aspects of radiation hardware4. The range of patient doses from individual procedures is highly variable, ranging from low or zero dose in electrophysiology studies to cumulative entrance air kermas of multiple Gy for certain complex coronary procedures such as interventions for chronic total occlusions. Indeed, in these latter procedures the entrance air kerma may exceed the threshold for skin injury erythema with levels in the range of radiotherapy fractions.
In the same article1, we reflected on the old public health adage that the first indications of toxicity from a technology often come from those who are occupationally exposed. We mentioned the illnesses suffered by the miners of Schneeberg in Germany due to radon gas exposure in the early part of the last century and those suffered by the so-called “radium girls” who painted the faces of watches with radium so that they could be read in the dark5. In terms of medical radiation, it was relatively early in its experience that occupational illnesses such as leukaemia and anaemia were noted, leading to the publication of safety standards as early as 1916. Occupational exposure in interventional cardiology is of particular concern and, unfortunately, many of us know of colleagues who have been significantly affected by this issue.
In the European Union, the current dose limits for occupational exposure are defined by a European Council Basic Safety Standards (BSS) Directive (2013/59/EURATOM)6. This is a directive which was required to be transposed into law in each member state by February 2018; it differs from a regulation, like the Medical Device Regulation or the General Data Protection Regulation, which automatically becomes law in the member states. The limit on the effective dose for whole body occupational exposure is defined in Article 9 of the directive as 20 mSv in any single year. This is well above the typical effective dose received by an interventional cardiologist of 2-4 mSv/year7. The limit on the equivalent dose for the lens of the eye is also 20 mSv in a single year (or 100 mSv in any five consecutive years). Importantly, this latter dose limit represents a sevenfold reduction in comparison with prior legislation. In Article 40, the directive also mandates classification of workers according to exposure doses. High-risk workers (Category A) are defined as those who are liable to receive an effective dose >6 mSv per year or an equivalent dose >15 mSv per year for the lens of the eye. Changes in the eye dose limit are important and affect interventional cardiologists in two ways. First, a higher proportion of interventional cardiologists will be classified as Category A workers. These workers are subject to closer scrutiny regarding fitness to work, including requirement for annual medical examination. Second, it is possible that selected, high-volume operators might exceed the cumulative dose threshold from clinical work over a five-year period.
In the current issue of EuroIntervention, the results of two randomised trials examining strategies for operator radiation exposure are reported8,9. Taken together, these papers represent a useful addition to the literature in this field. In the ESPRESSO trial, Remzi Anadol and colleagues examined the impact of a lead curtain attached to the underside of the ceiling-mounted lead shield and the use of the same curtain in association with a lead drape placed across the waist of the patient8.
In this single-centre, open-label study, both strategies were compared against a control group where only a ceiling-mounted lead shield was used. About half of the procedures were coronary angiography alone, and about half were coronary interventions. The main finding was that use of the shield-attached curtain plus an additional patient drape reduced operator radiation exposure but that the shield-attached curtain alone was only partially effective. The primary endpoint – the ratio of operator-to-patient radiation exposure – was 5.5 (4.1-7.5) versus 4.9 (3.3-6.7) versus 4.6 (3.3-5.8) for control, curtain only, curtain + drape respectively, p for across group comparison = 0.025. In terms of operator dose, the effective dose received was 13.0 (6.0-20.5) uSv versus 12.3 (6.1-21.0) uSv versus 9.0 (4.9-16.0) uSv, p for across group comparison = 0.002. The findings are largely confirmatory in relation to the use of lead drapes and build on the experience of other investigators in recent years10,11. It is somewhat surprising therefore that they remain largely underutilised in clinical practice. The findings also support, albeit to a lesser extent, the use of shield-attached lead curtains, which are part of the standard equipment in many laboratories.
In the second report, David Leistner and colleagues report the results of the RAMBO trial, which examined the use of biplane versus monoplane imaging in relation to operator radiation exposure in coronary angiography and intervention (mainly diagnostic angiography), also in the setting of a single-centre, open-label study9.
The main result was that operator effective dose measured at the level of the left arm was significantly higher in the biplane as compared with the monoplane group (median [interquartile range] 4 [1-13] uSv versus 2 [1-7] uSv, p<0.001). Fluoroscopy time was similar between the groups. The expected advantage with biplane imaging in terms of contrast reduction could be demonstrated. In many respects, and within the limits of a single-centre experience, this study too reinforces what we do in routine clinical practice: it suggests that biplane imaging is best reserved for selected indications (e.g., certain structural interventions); it is not the optimal modality for day-to-day coronary work in the cath lab. It should be noted that neither study accounted for the effect of personal protective equipment, which can reduce the effective dose received by the operator by a factor of around 20 (Table 1). Moreover, it might be argued that neither assessed a metric of image quality or diagnostic or therapeutic procedural efficacy, which should also be assessed in parallel to capture unexpected effects of any change in work practices.
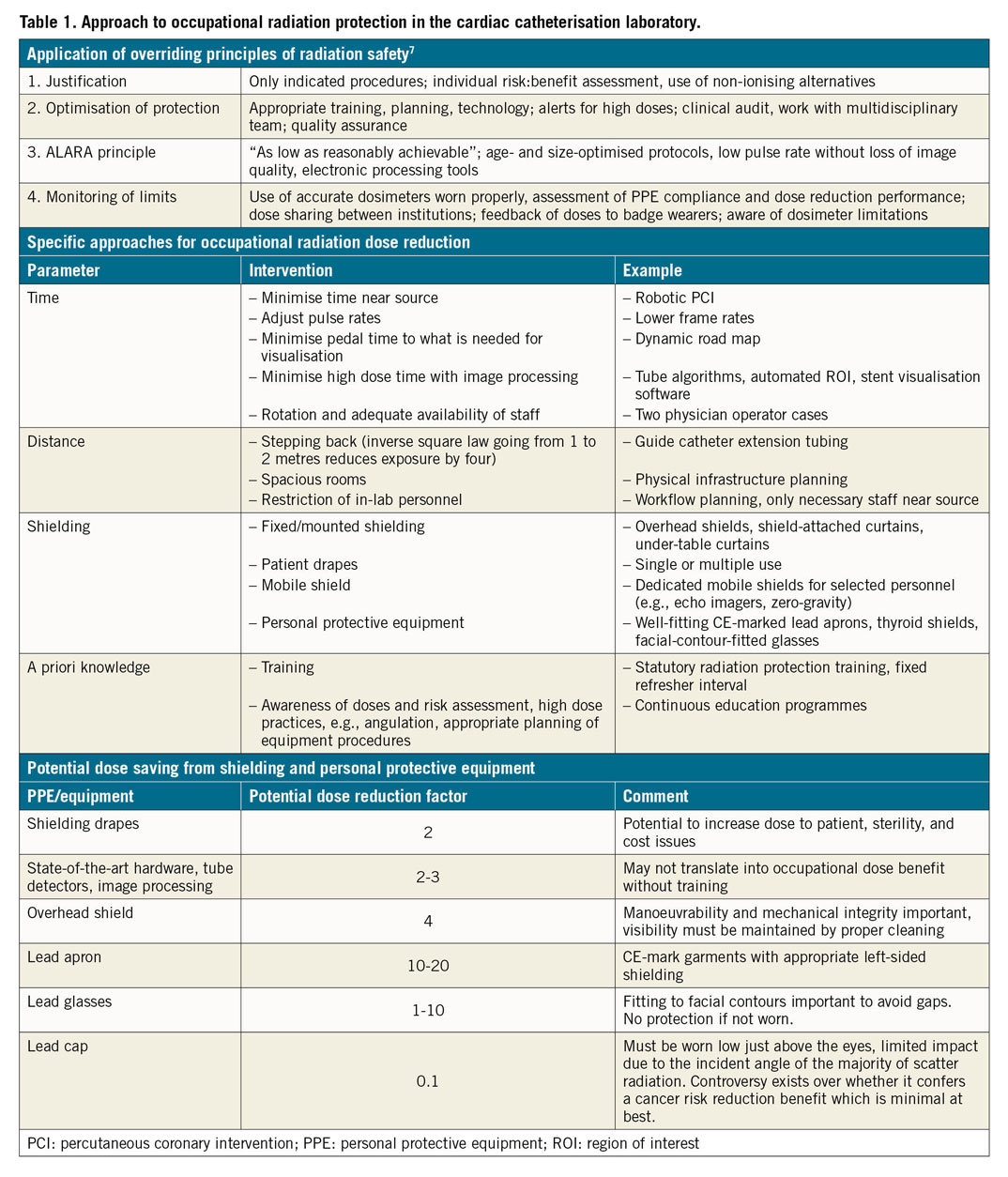
The trials reported address just some of a number of approaches which have recently been investigated for reduction of both patient and operator radiation exposure (Table 1). Adherence to the standard overriding principles of radiation protection remains a cornerstone of good practice. Additional dose reduction strategies can be considered under four categories: radiation time reduction, increasing distance from the source, enhanced use of shielding, and improved a priori knowledge. Some recent interest has centred on robot-assisted PCI12. While this reduces radiation exposure to the main operator, procedural efficiency may be compromised (increased procedure time) and the technology has not enjoyed widespread adoption. Studies have also investigated automated algorithms using partially X-ray attenuating plates which are controlled by a table-mounted operator-controlled tablet, or by eye-control technology13. This technology focuses radiation exposure to where the operator is looking on the screen while leaving the peripheral fields of view partially visible (e.g., so that the distal wire position may be monitored). While initial experiences are encouraging, further investigation including randomised trials on clinical fixed c-arm systems is warranted.
The long-term success of interventional cardiology for the diagnosis and treatment of coronary and structural heart disease will depend not only on high safety and efficacy of patient outcomes but also on sustainability in relation to the occupational health of catheterisation laboratory workers. Healthcare systems which rely on delivery of care by high-volume operators must improve the protection of their frontline catheterisation laboratory personnel. Approaches to improve occupational health must be multifaceted, taking into account both mental and physical wellbeing. Ongoing improvements in work practices relevant to occupational exposure to radiation are a critical component. The active engagement both of interventional cardiologists at an institutional level and of interventional professional societies at a national and international level is a sine qua non.
Conflict of interest statement
R.A. Byrne reports a research contract with CeloNova Biosciences with the institution of his prior employment (not related to the current work). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

