Abstract
Aims: The aim of this study was to test the radioprotection efficacy and comfort of newer bilayer barium sulphate-bismuth oxide composite (XPF) caps in an interventional cardiology setting.
Methods and results: Operators were randomly assigned to wear standard fabric (n=59), 0.3 mm (n=74), or 0.5 mm (n=64) lead-equivalent XPF caps. Radiation doses were measured by using dosimeters placed outside and underneath the caps. Wearing comfort was assessed at the end of each measurement on a visual analogue scale (VAS) (0-100, with 100 indicating optimal comfort). Procedural data did not differ between the XPF and standard groups. Mean standard, XPF 0.3 mm, and XPF 0.5 mm cap weights were 12.5 g, 118.4 g, and 123.7 g, respectively. VAS comfort ratings of the standard and XPF caps did not differ significantly (p=0.272). The mean radiation protection was 12.0%, 95% CI: 4.9-19.1% (standard caps, n=35), 91.5%, 95% CI: 87.4-95.6% (XPF 0.3 mm caps, n=45) and 97.1%, 95% CI: 92.5-100% (XPF 0.5 mm caps, n=44) (p≤0.001 for all group comparisons). Using the XPF caps, a cumulative total radiation dose reduction by almost factor 10 was evident (272 procedures, 22,310 μSv outside the XPF caps, 2,770 μSv inside the caps).
Conclusions: Lightweight XPF caps show comparable comfort to standard fabric caps, but provide substantial radiation protection during fluoroscopy-guided cardiac interventions.
Introduction
Over the last decades, the number of fluoroscopy-guided diagnostic and interventional procedures has dramatically increased1. However, along with the benefits of these technologies, there is great concern regarding radiation exposure, to both clinicians and patients. In particular, interventional cardiologists are exposed to the highest cumulative radiation among health professionals, and possible associations of cardiologists’ occupational radiation exposure with brain cancer, cataract and other diseases have been reported2-5. Thus, radiation exposure should be kept “as low as reasonably achievable (ALARA)”. The traditional personal radiation protection of interventional cardiologists generally includes an apron/thyroid shield and lead goggles. Noteworthy is the fact that no widely accepted and scientifically evaluated personal protection device for the head is currently available. Recently, bilayer barium sulphate-bismuth oxide composite (XPF) thyroid collars have been reported to be a lightweight alternative to standard 0.5 mm lead-equivalent thyroid collars and have provided superior radiation protection during fluoroscopy-guided interventions6,7. The two-layer design of barium sulphate and bismuth oxide was developed specifically to filter more effectively the low energy scatter radiation spectra that interventionalists typically encounter. The details have been reported elsewhere8.
A cap composed of this novel material is now commercially available, but no randomised controlled data exist evaluating its efficacy in an interventional cardiology setting. Therefore, the primary objective of the present trial was to compare the radiation attenuation provided by XPF caps (0.3 mm lead-equivalent and 0.5 mm lead-equivalent) in absolute and relative terms to a standard cap (fabric cap without radiation protection capabilities). The second objective was to assess the operator comfort wearing the standard fabric caps compared to the XPF protection caps.
Methods
This investigator-initiated, single-centre, prospective, randomised controlled trial was performed in the interventional cardiology suites of a tertiary care centre.
Institutional review board approval was obtained for this study which complied with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. All participating operators provided written informed consent. The study is registered at www.ClinicalTrials.gov (NCT01620658), and this article is written according to the reporting standards of the Consolidated Standards of Reporting Trials statement9. The manufacturer of the XPF radiation protection devices (BLOXR, Salt Lake City, UT, USA) provided the XPF and standard caps (same cap but without XPF protection layer) as well as the radiation detectors used in this study. The study was designed, the data were collected and analysed, and the manuscript was prepared exclusively by the study investigators, all of whom made the decision to submit the manuscript for publication and vouch for the accuracy and completeness of the data and analyses.
PROCEDURES
On the basis of the sample size power analysis, 197 operator-day measurements, obtained during 548 cardiac procedures requiring C-arm fluoroscopy, were performed over 151 days between August 2012 and September 2013. Procedure (type and duration of intervention, operator, access site, fluoroscopy time, dose-area product [DAP], and air kerma) data for each intervention were documented. All tubes had DAP meters indicating the summed cumulative DAP (in centigray-square centimetres). DAP is used as a surrogate measurement for the total amount of radiation energy delivered to the patient and, hence, also serves as a relative indication of the scatter dose to the operator.
PROTECTION DEVICES AND RANDOMISATION
Every morning, all operators scheduled to perform at least two interventional cardiology procedures during the same day were asked to participate and, after providing consent, were randomly assigned to wear standard (placebo) caps, 0.3 mm lead-equivalent or 0.5 mm lead-equivalent XPF caps by an independent member of the research department by using presealed envelopes with random group assignment numbers (Research Randomizer, version 3.0; http://www.randomizer.org). The experimental shielding material (bilayer barium sulphate-bismuth oxide composite [XPF]), is approved as a personnel protective shield (Food and Drug Administration 510[k] number K110900).
Ten standard, ten 0.3 mm lead-equivalent XPF and ten 0.5 mm lead-equivalent XPF caps of each size (small, medium, large) were weighted by using a scale with an accuracy of ±0.01 g according to the manufacturer (EL-2000S; Setra Systems, Inc., Boxborough, MA, USA). Measurement of radiation exposure radiation doses in each procedure was performed with two optically stimulated luminescence dosimeters (Luxel®+; Landauer, Glenwood, IL, USA) placed side by side - one outside the cap and one underneath the cap at a standardised left forehead position using premounted detector pockets. Radiation dose (in millirems) was reported as a shallow dose equivalent, which applies to the external exposure of the skin or extremity at a tissue depth of 0.007 cm (7 mg/cm2) averaged over an area of 1 cm2 and converted to microsieverts by multiplication with factor 10 (equivalent dose). The radiation protection (the predefined primary outcome) was calculated as specified later. The dosimeters were calibrated with x-ray beams identical to those used in clinical practice, and the sensitivity threshold for the dosimeters was approximately 10 μSv. After randomisation, each operator received either a standard, a 0.3 mm lead-equivalent or a 0.5 mm lead-equivalent XPF cap with two radiation detectors mounted by the study personnel at the start of the operator’s shift, and cumulative radiation exposure was measured during all procedures within that shift. At the end of the operator’s shift, the operator returned the dosimeters to a study box that was positioned next to the cathlab, and a study nurse ensured that all detectors were returned promptly. All dosimeters (used and unused) were stored in the same box to control for any background radiation. After detectors had been collected for approximately 25 study days, the used dosimeters were sent to Landauer, and radiation exposure readings were performed blinded with respect to the dosimeter study group allocation (standard vs. XPF 0.3 mm vs. XPF 0.5 mm) and position (outside vs. underneath the cap).
OPERATOR COMFORT ASSESSMENT
After completion of the last procedure on each day, the operators were asked to rate the comfort of wearing the cap on a scale from 0 (unbearably heavy, badly fitting) to 100 (very light, well fitting).
STATISTICAL ANALYSES
The radiation protection (radiation dose reduction) expressed as a percentage was calculated by subtracting radiation measured underneath the cap from radiation measured outside the cap and then dividing this difference by the product of radiation measured outside the cap multiplied by 100. The cumulative radiation doses were defined as the summation of all correspondent equivalent doses measured. Summary values were presented as mean±standard deviation (SD), mean and 95% confidence interval (CI) or median±interquartile range (IQR). Normality was tested by using the Kolmogorov-Smirnov test. For normally distributed data, we used a two-sided unpaired Student’s t-test, and for non-normally distributed data we used the Kruskal-Wallis test, non-parametric Mann-Whitney U test, or Wilcoxon signed-rank test, as appropriate. For the analysis of comfort, the respective percentage was dichotomised into high comfort (i.e., values ≥90%) or low to medium comfort (values <90%). A logistic regression model with operators as covariate was then used to compare the likelihood of high comfort among the groups. Spearman rho analyses were performed to test for correlations between radiation measurements and cumulative fluoroscopy time/cumulative DAP. A logistic regression model was also used to test for an association between negative radiation measurements and cumulative DAP/fluoroscopy time. A p-value of less than 0.05 was considered to indicate a significant difference. All analyses were performed by using statistical software (IBM SPSS Statistics for Windows, Version 22.0; IBM Corp, Armonk, NY, USA). On the basis of previously reported dose reduction rates6,7, we estimated that approximately 50% of the outside radiation measurements would be below the detector threshold, and that the standard deviation of the primary endpoint would be approximately 30%. The ANOVA-based three-group sample size calculation with Bonferroni correction indicated that a total of 132 measurements (44 in each group) would be sufficient to demonstrate an arbitrarily set 20% difference in radiation protection (effect size) among the groups with a statistical power of 80% and an alpha level of 1.7% (superiority trial design). The null hypotheses were that the XPF 0.3 mm, XPF 0.5 mm and standard caps would show no difference with regard to radiation protection. The alternative hypotheses were that the XPF or standard caps would provide superior or inferior radiation protection as compared to each other.
Results
Figure 1 displays the study and analysis flow. All data sets (n=197) were included in the comfort analysis. In two cases (one in the standard group, one in the XPF 0.3 mm group), no detector results at all were available and, in 71 (36%) of the 197 measurements, the radiation exposure outside the cap was below the detector threshold. Radiation exposure moderately correlated to the cumulative fluoroscopy (r=0.349, p<0.001) and cumulative DAP (r=0.416, p<0.001) (Spearman). Negative readings (radiation exposure below detector threshold) were associated with a lower cumulative DAP only but not with cumulative fluoroscopy time (p=0.014 and p=0.420, respectively; logistic regression analysis). These data sets were excluded from further radiation protection analysis, resulting in 124 radiation protection measurements (35 in the standard group, 45 in the XPF 0.3 mm group and 44 in the XPF 0.5 mm group) during a total of 375 cardiac procedures performed by four operators. Two operators participated significantly more than average, finally acquiring 114 of the 124 data sets included. No imbalance with regard to group allocation was detectable across operators (p=0.705, chi-square test).
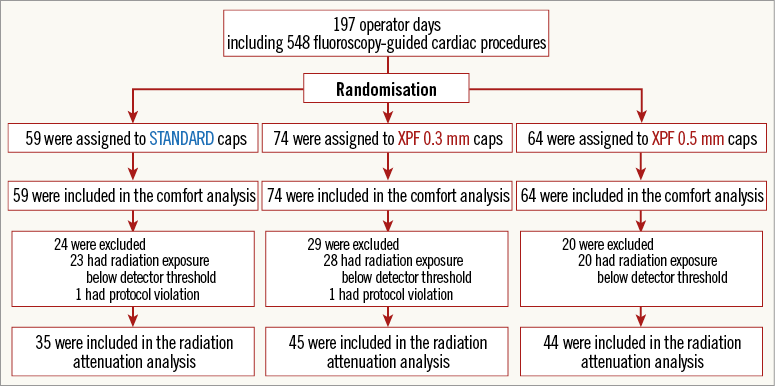
Figure 1. Study and analysis flow chart. A total of 197 operator-day measurements obtained during 548 fluoroscopy-guided cardiac interventions were performed with randomised allocation of the operators to standard fabric, XPF 0.3 mm lead-equivalent or XPF 0.5 mm lead-equivalent caps.
Table 1 displays and compares the procedure-specific data of the procedures included in the radiation protection analysis. As outlined, no significant difference between the standard and XPF groups was detectable with regard to the single procedures themselves as well as to the cumulative data for all procedures included in one radiation measurement.
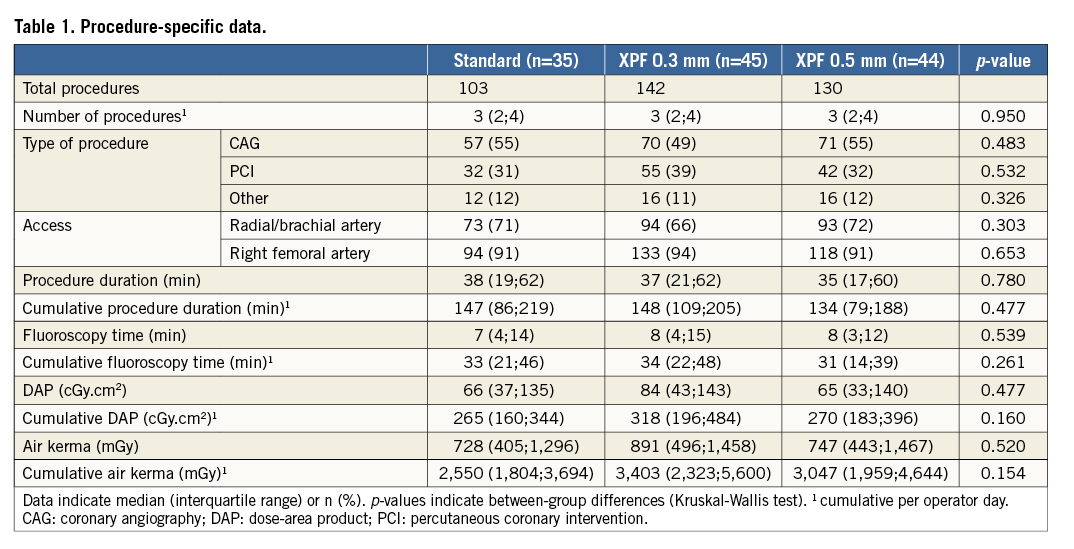
The total radiation dose measured outside the caps during the 375 procedures was 33,020 μSv. The mean radiation doses measured outside the caps did not differ significantly between the standard and XPF groups (132 μSv, 95% confidence interval [CI]: 21-243 μSv in the standard group, and 89 μSv, 95% CI: 54-124 μSv in the XPF groups; p=0.926, Mann-Whitney U test) as well as between the XPF 0.3 mm and 0.5 mm groups themselves (118 μSv, 95% CI: 54-183 μSv vs. 59 μSv, 95% CI: 33-85 μSv; p=0.068, Mann-Whitney U test). Using the XPF caps, a cumulative total radiation dose reduction by almost factor 10 was evident (272 procedures, 22,310 μSv outside the XPF caps, 2,770 μSv inside the caps). Absolute mean (95% CI) and median (IQR) values are presented in Table 2. Figure 2 displays the radiation protection provided by the standard, XPF 0.3 mm, and XPF 0.5 mm caps. The median radiation protection provided by the standard, the XPF 0.3 mm, and XPF 0.5 mm caps was 0% (interquartile range [IQR] 0 to 24%), 100% (IQR 85% to 100%), and 100% (IQR 100% to 100%), respectively. Accordingly, both types of XPF caps provided significantly better radiation protection than the standard caps (p<0.001 for both group comparisons, Mann-Whitney U test), and the 0.5 mm XPF caps provided significantly better radiation protection than the 0.3 mm XPF caps (p=0.001, Mann-Whitney U test). The mean radiation protection was 12.0%, 95% CI: 4.9-19.1% (standard caps), 91.5%, 95% CI: 87.4-95.6% (XPF 0.3 mm caps), and 97.1%, 95% CI: 92.5-100% (XPF 0.5 mm caps) (p≤0.001 for all group comparisons, Kruskal-Wallis test).
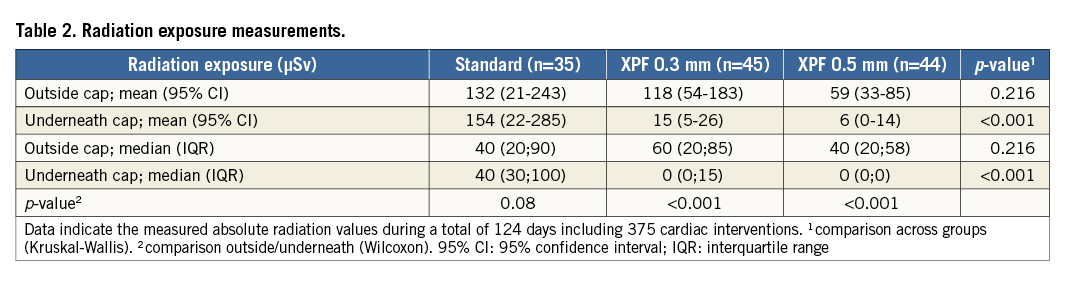
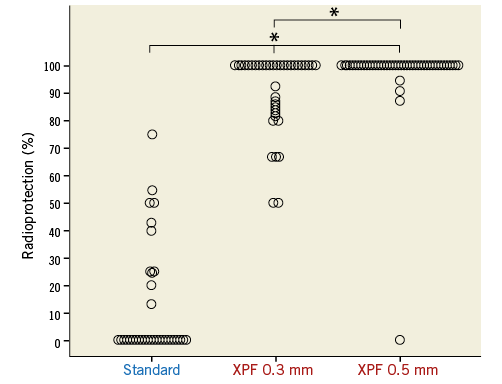
Figure 2. Dot plot showing the radioprotection provided by the standard fabric (n=35), XPF 0.3 mm lead-equivalent (n=45), and XPF 0.5 mm lead-equivalent (n=44) caps. * indicates a p-value ≤0.001 for between-group differences (Kruskal-Wallis test).
OPERATOR COMFORT ASSESSMENT
Mean standard, XPF 0.3 mm, and XPF 0.5 mm cap weights were 12.5 g (95% CI: 12.5-12.6 g), 118.4 g (95% CI: 116.7-120.3 g), and 123.7 g (95% CI: 122.9-124.6 g), respectively (p<0.001 comparing the standard vs. XPF group, unpaired t-test). Figure 3 displays the mean weight of the different caps stratified by size.
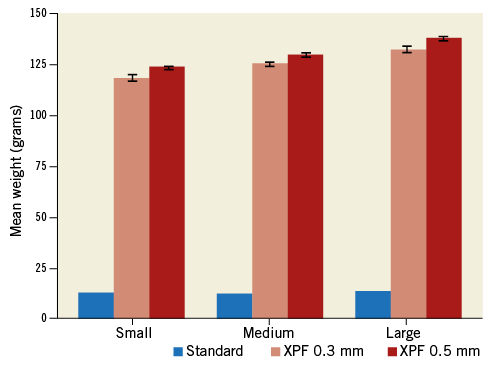
Figure 3. Bar chart showing mean cap weight and 95% confidence intervals (whiskers) of 10 standard fabric, XPF 0.3 mm lead-equivalent, and XPF 0.5 mm lead-equivalent caps according to size (small, medium, large).
The operator comfort rating on the VAS (score range, 0-100), with higher numbers indicating better comfort, was obtained in all cases and for all operators, resulting in comfort ratings for a total of 59 standard caps and 138 XPF caps (74 in the XPF 0.3 mm and 64 in the XPF 0.5 mm group). The overall median duration the caps were worn was 116 minutes (IQR 33-178 min): the standard caps were worn 110 minutes (IQR 75-184 min), the XPF 0.3 mm caps 127 minutes (IQR 79-183 min), and the XPF 0.5 mm caps 111 minutes (IQR 67-170 min) (p=0.567, Kruskal-Wallis test).
The overall median comfort rating was 90 out of 100 on the VAS (IQR 90-90). Comfort ratings for the XPF caps (VAS 90, IQR, 90-90) were not significantly lower compared with the comfort ratings for the standard caps (VAS 90, IQR 90-100; p=0.272, Mann-Whitney U test). In the logistic regression model with and without operators as covariate, the estimated odds ratio of a high level of comfort (VAS ≥90) between the standard and XPF groups did not differ significantly (0.69, 95% CI: 0.16-3.02; p=0.621, and 0.87, 95% CI: 0.22-3.40; p=0.842, respectively).
Discussion
Recent reports have raised increasing concerns that brain cancer may be an occupational risk of interventional cardiologists and radiologists due to chronic occupational radiation exposure10-15. Although direct evidence in fluoroscopy is lacking due to sample size limitation, limited follow-up time, and lack of focused research, ionising radiation is an established cause of brain cancer16-18. In interventionalists, the left side of the head is known to be more exposed to radiation than the right due to the usual interventional room layout with the x-ray tube positioned on the operator’s left19. Therefore, recent reports on clusters of left-sided brain tumours suggest a biologically plausible connection to the chronic cumulative radiation exposure10-12. In high-volume centres, interventionalists may perform hundreds of procedures each year with an annual exposure equivalent to around 5 mSv below the apron19. Though ceiling-suspended screens may offer some protection20-23, no widely accepted and scientifically evaluated personal protection device for the head is currently available. Therefore, the reported 10-fold to 20-fold higher head organ dose (compared to the protected body areas beneath an apron) with a radiation dose of 100 μSv per single ablation procedure and an annual head exposure in the range of 20-30 mSv is of specific concern24,25. New data from exposed interventional cardiologists and nurses suggest that lens opacities and cataracts occur frequently and at doses far lower than previously believed26-28.
Our study results confirm the fact that the heads of interventional cardiologists are exposed to a substantial cumulative radiation dose. During the 548 cases monitored in our study, a procedure volume reached by many interventionalists within one to two years at high-volume centres, the cumulative radiation exposure of the head was greater than 33 mSv despite standard measures to limit radiation exposure (e.g., by using ceiling-suspended transparent leaded plastic shields). Given the limitation of the detector threshold, the real overall cumulative radiation exposure was likely to be even higher than the observed one. This highlights the need for optimal personal radiation protection. The observation that the cumulative fluoroscopy time and DAP (indicating more complex cases) correlates only weakly to the effective radiation exposure measured might also indicate that one’s radiation exposure can be substantially altered by personal protection efforts. Our prospective, randomised controlled study of caps made of a disposable, lightweight, lead-free material (XPF) provides evidence for an effective way to optimise individuals’ head radiation protection. Both XPF 0.3 mm and 0.5 mm lead-equivalent caps reduced the radiation exposure by approximately 90% and showed comparable comfort to standard fabric caps with an average VAS comfort rating higher than 90. Previously tested protection caps also showed substantial protection against the significant amount of radiation that reaches the operator’s head; however, a cap weight of approximately 1,140 g may induce discomfort and debilitating musculoskeletal disorders in the long term and may have prevented its widespread use in clinical practice29,30. Therefore, the XPF caps represent a reasonable alternative, given their average weight of less than 125 g. Undoubtedly, combined with other previous innovations to reduce radiation exposure of the head, including conventional suspended shields23, protection cabin24, protection shields with a complex overhead motion system31, and/or protective sterile surgical drapes32,33, a dramatic dose reduction for the operators is potentially achievable. While previous reports focused on creating awareness of patient hazards of cumulative exposure to ionising radiation from diagnostic and therapeutic (cardiac) imaging procedures1,34-36, few novel efforts have been put forward which try to minimise operators’ radiation exposure.
As much as medicine and industry are focused on creating new technologies and therapies to advance patient care, the time has come to ensure that these methods of delivery of care are safer for all those involved37,38. Though limited by its single-centre design, the particular strengths of the present study are its prospective randomised design and the broad spectrum of diagnostic and interventional procedures, ensuring that our results are applicable for most fluoroscopy-guided cardiac procedures. Our results on the newly developed lightweight protection caps composed of a bilayer barium sulphate-bismuth oxide composite are therefore encouraging and may facilitate their implementation and acceptance among operators in order to optimise personal radiation protection. We did not analyse the cost-effectiveness of the XPF caps compared with other specific protection devices (i.e., heavy lead cap, suspended ceiling or protection cabin). However, the undeniably substantial cumulative radiation exposure of the operators should alleviate any cost concerns precluding less than optimal radiation protection.
In addition to the single-centre design and limited number of operators involved, some other limitations of our study are noteworthy. First, using a simple randomisation algorithm, an imbalanced group allocation was observed in our study. Though procedure parameters (Table 1, Table 2) indicate that the randomisation process was nevertheless effective, a block randomisation might have been more appropriate. Furthermore, the use of more sensitive digital online radiation measurement devices would allow for single procedure readings and a potentially better comparison. In summary, this randomised controlled study demonstrates that lightweight XPF caps show comparable comfort to standard fabric caps, but provide substantial radiation protection during fluoroscopy-guided cardiac interventions.
| Impact on daily practice Recent reports have raised increasing concerns that brain cancer may be an occupational risk for interventional cardiologists and radiologists due to chronic occupational radiation exposure. The present randomised controlled study (n=197) demonstrates that lightweight XPF caps show comparable comfort to standard fabric caps, but provide substantial radiation protection (>90%) during fluoroscopy-guided cardiac interventions. Therefore, protection caps made of this novel bilayer barium sulphate-bismuth oxide composite may have the potential to optimise radiation protection and operator comfort in the future. |
Acknowledgements
We thank all participating interventional operators for their dedication to research and the institution’s Department of Research and Outcome team for help in data collection. We thank BLOXR (Salt Lake City, UT, USA), the manufacturer of the XPF protection devices, for providing the caps and radiation detectors used in this study.
Funding
This work was supported by the Department of Medicine, University Hospital Basel, Basel, Switzerland (VWFAWF-Pool FO135400 to HU).
Conflict of interest statement
H. Uthoff has received non-financial support from BLOXR and reimbursement of expenses for travel to the International Symposium on Endovascular Therapy 2013 from BLOXR. The other authors have no conflicts of interest to declare.

