
Introduction
Significant geographic barriers limit timely access to percutaneous coronary intervention (PCI) in many regions of the world. One proposed means to increase PCI access is telestenting, defined as the performance of PCI using a combination of robotics and telecommunications by an operator in a separate geographic location from the patient1. In the present study, the feasibility of long-distance telestenting was tested sequentially in ex vivo and in vivo models.
Methods
The ex vivo telestenting model utilised an endovascular simulator (ANGIO Mentor™; Simbionix, Littleton, CO, USA) into which guide catheters, guidewires and balloon catheters were inserted. Advancement, retraction, and torqueing of these devices was detected by sensors in the simulator and displayed on a monitor as corresponding movements of virtual devices within the endovasculature of a simulated patient.
In the in vivo telestenting model, PCI procedures were conducted on five consecutive 16-week-old, 50 kg female Yorkshire swine. The Institutional Animal Care and Use Committee of the West Michigan Regional Laboratory (Grand Rapids, MI, USA) approved the study protocol. Experiments were performed according to the committee’s guidelines. Induction of anaesthesia was achieved with Telazol® (6.6 mg/kg), xylazine (2.2 mg/kg), and ketamine (33 mg/kg). Isofluorane (4% inhalant) was administered for general anaesthesia. Femoral arterial access was obtained with a 7 Fr sheath.
For ex vivo and in vivo cases, a cardiology fellow manually engaged a guide catheter into a target coronary artery, positioned a guidewire at the tip of the guide catheter, and loaded the guidewire and balloon/stent catheter onto a bedside robotic drive (CorPath GRX; Corindus Vascular Robotics, Waltham, MA, USA). An offsite interventional cardiologist performed all subsequent manipulations of the guide catheter, guidewire, and balloon/stent catheter using robotic controls connected over a virtual private network (VPN) to the robotic drive with hardware firewalls in place to secure and isolate device data within the network. In the ex vivo model, the VPN was entirely on the study institution’s internal network. In the in vivo model, a portion of the VPN utilised commodity internet. Each side was synchronised using a grandmaster clock and global positioning system antenna. Live fluoroscopy images and haemodynamic waveforms were transmitted to the interventional cardiologist’s location.
The outcome measure for each telestenting attempt was procedural success, defined as the successful robotic manipulation of guidewires and balloon/stent catheters necessary to achieve stent deployment at the target site without conversion to a manual operation. Target sites for stent deployment in the ex vivo model were defined as the location of a severe stenosis within the virtual target artery. Target sites for stent deployment in the in vivo model were defined within each target artery as specific locations identifiable by anatomic landmarks such as side branches.
Results
In May 2018 the feasibility of telestenting was tested in the ex vivo model (Figure 1). The interventional cardiologist was located 7.4 kilometres from the virtual patient and telestenting was attempted in two consecutive cases at three distinct anatomic target sites. In each case, the guidewire was robotically delivered and balloon/stent catheters were robotically advanced to the target lesion to facilitate virtual stent deployment. Procedural success was achieved at all three target sites.
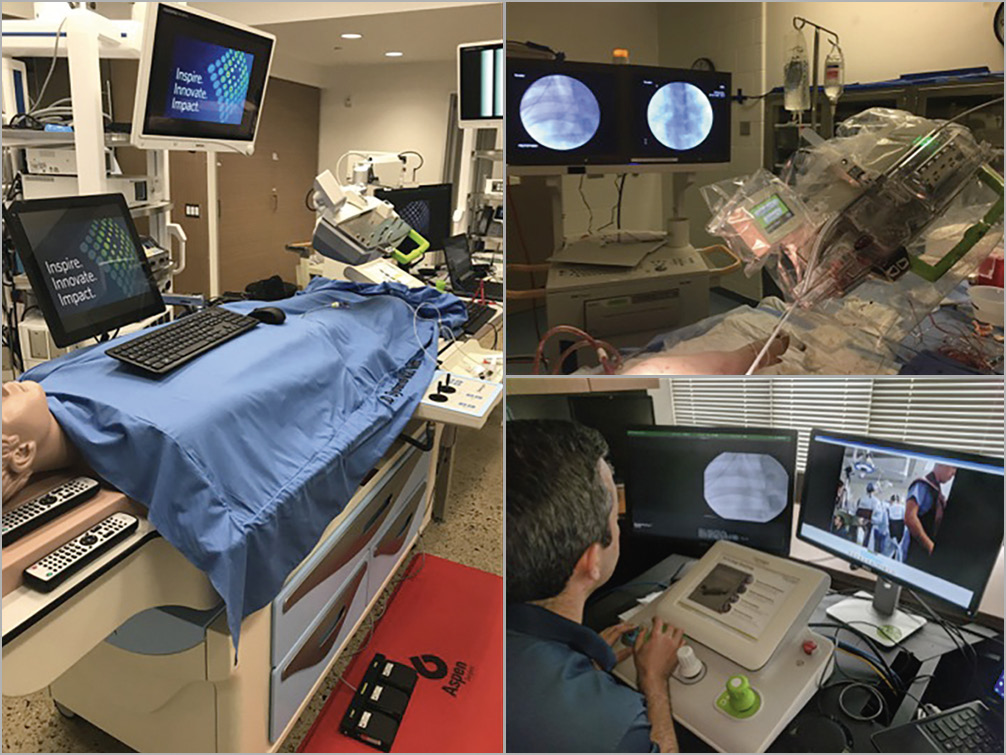
Figure 1. Ex vivo and in vivo models of telestenting. The feasibility of telestenting was tested ex vivo at a cardiovascular simulation laboratory (left) and in vivo using a porcine model at an animal laboratory (upper right). Ex vivo and in vivo robotic telestenting was performed by an offsite interventional cardiologist (lower right).
In June 2018 the feasibility of telestenting was tested in vivo. During the first two cases, the interventional cardiologist was located 6.6 kilometres from the animal laboratory and telestenting was attempted at four anatomic target sites (Figure 1). In the next three cases, the interventional cardiologist was located 166 kilometres from the animal laboratory and telestenting was attempted at an additional four anatomic target sites. In all cases, the guidewire was robotically delivered and balloon/stent catheters were robotically advanced to the target lesion to facilitate stent deployment. Procedural success was achieved at eight of eight attempted target sites. No procedural complications occurred.
Discussion
By leveraging existing robotic and commodity telecommunications technologies, the present preclinical study is the first to demonstrate the feasibility of robotic telestenting over long geographic distances. This was initially demonstrated in an ex vivo model where an interventional cardiologist successfully performed telestenting on a PCI simulator despite being 7.4 kilo-metres away from the simulated patient. These successful ex vivo results led to the testing of telestenting in an in vivo porcine model over distances of 6.6 kilometres and 166 kilometres. Telestenting was successfully performed in vivo in eight of eight attempted coronary target sites, thereby demonstrating the feasibility of telestenting in vivo over long geographic distances. These promising preclinical results likely represent a critical step in the eventual development of studies to test the safety and feasibility of telestenting in humans as a means to address barriers to PCI access.
Limitations
The need for a second operator is a limitation inherent to telestenting. Indeed, the need for the receiving hospital to have trained personnel, PCI supplies, a robotic drive, and a robust network capable of facilitating low-latency signal transmission may limit real-world applications. The present cases were successfully performed without signal loss, yet the risk of sudden breakdown in robotic signal transmission is a limitation that will need to be addressed. Considerable technological logistics were required to connect the robotic components over a network. Future advances may eventually enable efficient connectivity on an as needed basis. It should be noted that, although the ex vivo simulation cases in this study were conducted over the study institution’s private network, the in vivo telestenting cases were successfully performed while relying on commodity internet for a portion of the connection. Whereas demonstrating that telestenting is feasible when performed over commodity internet might increase the generalisability of the present observations, the use of commodity internet, rather than a private network, probably introduces additional concerns regarding security and consistency of network performance, especially with regard to the potential detrimental impact of internet traffic on network speed. Finally, additional studies are needed to explore the impact of robotic command signal latency and whether telestenting over distances >166 kilometres would introduce prohibitive signal latency. The successful performance of transatlantic telerobotic cholecystectomy in 2001 suggests that telestenting over much greater distances may be feasible2.
Conclusions
Using ex vivo and in vivo experimental models, this study demonstrates for the first time the feasibility of robotic telestenting over long geographic distances. These preclinical results support the development of future studies to test the safety and feasibility of telestenting in humans.
|
Impact on daily practice Now that robotic telestenting has been demonstrated to be technically feasible in this preclinical research, future studies will probably be performed to test the safety and feasibility of telestenting in humans. If developed further, telestenting may eventually be tested as a means to overcome barriers to PCI access. |
Acknowledgements
The authors would like to thank Jacob Gonser, John Doneth, and Dr Andrew Kalafut for their technical assistance in the simulation and animal laboratories.
Funding
This work was supported by the Frederik Meijer Heart & Vascular Institute of Spectrum Health (Grand Rapids, MI, USA) and by Corindus Vascular Robotics (Waltham, MA, USA).
Conflict of interest statement
R. Madder reports grants and personal fees from Corindus Vascular Robotics, during the conduct of the study. S. VanOosterhout, A. Mulder, J. Bush, S. Martin, A. Rash, J. Tan, and J. Parker report grants from Corindus Vascular Robotics, during the conduct of the study. Y. Li, N. Kottenstette, and P. Bergman report being employees of Corindus Vascular Robotics, during the conduct of the study. B. Nowak reports grants from Spectrum Health, during the conduct of the study.

