
Abstract
Aims: The present study explores the feasibility of telestenting, wherein a physician operator performs stenting on a patient in a separate physical location using a combination of robotics and telecommunications.
Methods and results: Patients undergoing robotic stenting were eligible for inclusion. All manipulations of guidewires, balloons, and stents were performed robotically by a physician operator located in an isolated separate room outside the procedure room housing the patient. Communication between the operating physician and laboratory personnel was via telecommunication devices providing real-time audio and video connectivity. Among 20 patients who consented to participate, technical success, defined as successful advancement and retraction of guidewires, balloons, and stents by the robotic system without conversion to manual operation, was achieved in 19 of 22 lesions (86.4%). Procedural success, defined as <30% residual stenosis upon completion of the procedure in the absence of death or repeat revascularisation prior to hospital discharge, was achieved in 19 of 20 patients (95.0%). There were no deaths or repeat revascularisations prior to hospital discharge.
Conclusions: To the best of our knowledge, the present study is the first to explore the feasibility of telestenting. Additional studies are required to determine if future advancements in robotics will facilitate telestenting over greater geographic distances.
Introduction
Percutaneous coronary intervention (PCI) has traditionally been a manual procedure wherein a physician operator advances, retracts, and torques interventional devices in the coronary arteries by hand while standing adjacent to the patient1. A robotic system for performing PCI has recently been introduced, which allows an operator to perform stenting while seated at robotic controls located in the corner of the catheterisation laboratory2-4. To date, all coronary stenting procedures, whether conducted manually or robotically, have been performed by an operating physician located in the same procedure room as the patient.
Given that robotic PCI only requires the operator to be present at the controls but not necessarily at the patient’s bedside, it is conceivable that robotics may facilitate the performance of telestenting, wherein a physician operator performs PCI on a patient in a separate physical location. If telestenting were developed into a procedure capable of being performed over long geographic distances, it could be applied to improve access to PCI in medically underserved regions and might represent a novel alternative to inter-hospital transfer for primary PCI in patients with ST-segment elevation myocardial infarction (STEMI). Prior to pursuing such applications however, it must first be demonstrated that it is feasible for a physician to perform PCI from outside the confines of the procedure room. The present study was designed to assess the feasibility of this concept by: 1) removing the physician operator from the procedure room housing the patient; and 2) having the physician operator perform PCI from behind the closed doors of an isolated separate room using a combination of robotics and telecommunication devices.
Methods
STUDY POPULATION
The REMOTE-PCI study was a single-centre prospective observational study performed at the Frederik Meijer Heart & Vascular Institute (Spectrum Health, Grand Rapids, MI, USA). The study complied with the Declaration of Helsinki and the protocol was approved by the local institution review board. All participants provided written informed consent. Study personnel sought consent from patients referred for clinically indicated coronary angiography and possible PCI. Consenting patients were eligible for inclusion if subsequent invasive angiography demonstrated a severe stenosis requiring PCI and the target lesion was deemed suitable for treatment using a robotic approach. The decision regarding performance of PCI and the suitability for robotics was at the discretion of the operating physician. Patients were excluded from participation if any of the following criteria were present: 1) haemodynamic instability; 2) emergent need for PCI, including emergent primary PCI for STEMI; 3) cardiogenic shock; 4) severe multivessel coronary artery disease; 5) target lesion located in a coronary artery bypass graft; or 6) ejection fraction <35%.
ROBOTIC SYSTEM
Once the patient was deemed eligible for study inclusion, the operating physician manually engaged a guide catheter into the target vessel. All subsequent interventional procedures, including advancement and retraction of guidewires, angioplasty balloon catheters, and stent catheters, were performed using a robotic system (CorPath® 200; Corindus Vascular Robotics, Waltham, MA, USA). No alterations were made to the commercially available robotic system for this study. As previously described, the robotic system consists of a bedside robotic arm and a mobile interventional cockpit3,4. The robotic arm, which comprises a drive unit and an overlying sterile cassette, places axial and rotational forces on guidewires, balloon catheters, and stent catheters. The drive unit is controlled by buttons and two joysticks housed within the interventional cockpit. These controls allow precise movements of guidewires, balloons, and stents by increments as small as 1 mm proximally or distally. The interventional cockpit also contains two monitors showing real-time fluoroscopy and cineangiography images, along with a third monitor displaying real-time haemodynamic and electrocardiographic data.
LOCATION OF ROBOTIC CONTROLS AND DESCRIPTION OF TELECOMMUNICATIONS
The interventional cockpit, which is conventionally positioned in the corner of the catheterisation laboratory a few feet from the patient3,4, was removed from the procedure room housing the patient and placed behind the closed doors of an isolated separate room having no direct line of visual or audio contact with the patient or personnel in the catheterisation laboratory. Once in this position, the cockpit was located approximately 55 feet from the patient. The only means of communication between the operating physician and the individuals in the catheterisation laboratory was via telecommunication devices (TelePresence® VX Clinical Assistant; Cisco Systems Inc., San Jose, CA, USA) connected to each other through a Wi-Fi network. The telecommunications equipment provided real-time audio and, for most cases, video connectivity between the two locations. When available, the video connectivity allowed the patient and catheterisation laboratory staff members to see the physician operator on a monitor. Similarly, the video connectivity allowed the operator to have visualisation of the patient and the catheterisation laboratory. The operating physician was able to manipulate the video camera in the catheterisation laboratory using remote control.
PHYSICIAN OPERATORS AND CATHETERISATION LABORATORY PERSONNEL
The physicians performing robotic PCI as part of this study had been previously trained in robotic PCI and had considerable experience of performing robotic PCI in routine clinical practice prior to the commencement of this study. Robotic PCI training, which is required by the manufacturer prior to clinical use, consisted of a didactic learning session, performance of hands-on training with a bench-top model, passing a written exam, and completion of five proctored robotic PCI cases. Prior to study onset, physician operators in this study had each performed >25 robotic PCI procedures.
For all cases in this study, the physician operator performed the PCI while seated at the controls in the isolated room. As is standard with all interventional procedures at the study institution, a nurse circulator and scrub technician were present in the catheterisation laboratory with the patient for the duration of the procedure. The nurse provided medications as ordered by the operating physician and obtained interventional devices upon request. The scrub technician loaded and removed all interventional devices on the robotic arm, inflated angioplasty and stent balloons, provided movements of the fluoroscopy camera and operating table, and controlled the fluoroscopy and cineangiography pedals, all under the direction of the operating physician via telecommunications. The telecommunication devices enabled the operating physician to provide real-time verbal instructions to the scrub technician regarding the performance of these actions and also allowed the operating physician to monitor these actions visually as they were conducted. As a safety precaution, a second interventional cardiologist wearing proper sterile surgical attire was present in the procedure room with the patient for the duration of the PCI. This second physician, who served as a “safety net” in the event that an emergent need arose for a physician to be present at the bedside, did not manipulate guidewires, balloon catheters, or stent catheters in any of the cases.
STUDY OUTCOMES
The two pre-specified primary outcome measures of interest in this study were procedural success and technical success. Technical success, which is reported on a per lesion basis, was defined as successful intracoronary advancement and retraction of guidewires, angioplasty balloons, and stents by the robotic system without conversion to manual operation. Procedural success was defined as <30% residual stenosis upon completion of the procedure in the absence of death or repeat revascularisation prior to hospital discharge. Secondary outcome measures included death or repeat revascularisation prior to hospital discharge. Procedural time for each PCI was recorded as the time from initial robotic manipulation of the guidewire to robotic removal of the guidewire at the completion of the procedure. Measures of radiation exposure, including fluoroscopy time and air kerma, were recorded for each procedure.
STATISTICAL METHODS
Descriptive statistics were used to summarise baseline characteristics and outcome measures. Normally distributed continuous variables are shown as mean±standard deviation. Non-normally distributed continuous variables are shown as medians and median absolute deviations. Categorical variables are shown as counts and frequencies.
Fluoroscopy time and air kerma were compared in patients undergoing telestenting to those of a control group. Using propensity score matching, the control group was selected from a pool of 50 consecutive patients undergoing standard bedside robotic PCI with radial artery access at the study institution. Propensity score matching was performed using R (v3.2.0) making use of the MatchIt package (v2.4-21)5. The following variables were included in a logistic regression model to estimate the propensity scores: patient weight, number of lesions treated, ACC/AHA lesion classification, and target vessel. The logit of the propensity score was used as the distance measure for matching and the caliper width was set to be equal to 0.2 of the standard deviation of the logit of the propensity score6. Nearest-neighbour matching without replacement was used to match case and control patients. In this manner, suitable matches were found for all study patients. All other statistical analyses were performed using the R statistical software environment, version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
STUDY POPULATION
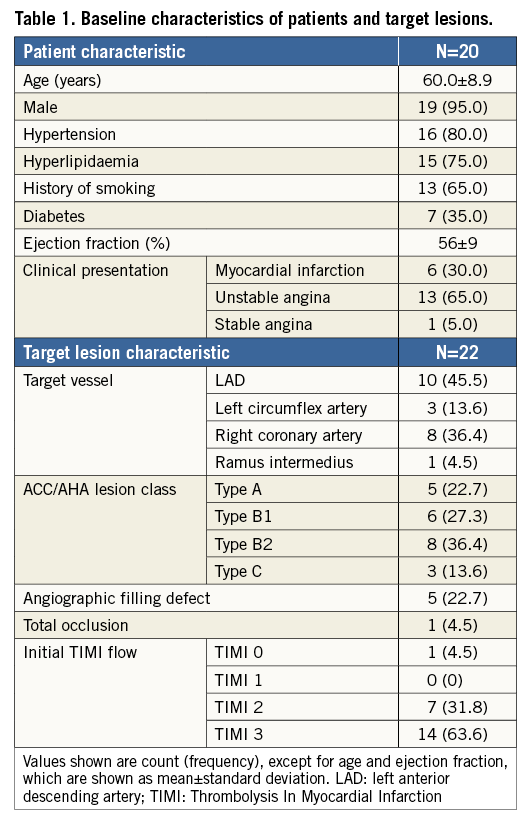
Between December 2014 and September 2015, a total of 20 patients consented to participate, met all inclusion criteria, and underwent attempted PCI according to the study protocol. The baseline characteristics of the study population are summarised in Table 1. Among the 20 patients, the clinical presentation was myocardial infarction in six (30.0%), unstable angina in 13 (65.0%), and stable symptoms in one (5.0%).
TARGET LESIONS
A total of 22 target lesions underwent attempted PCI. The characteristics of target lesions are presented in Table 1. Target lesions were located in the left anterior descending artery in 10 (45.5%) cases, right coronary artery in eight (36.4%) cases, left circumflex artery in three (13.6%) cases, and ramus intermedius artery in one (4.5%) case. An angiographic filling defect consistent with thrombus was present at the site of five (22.7%) target lesions. In one case, the target lesion was a total occlusion.
Procedural details
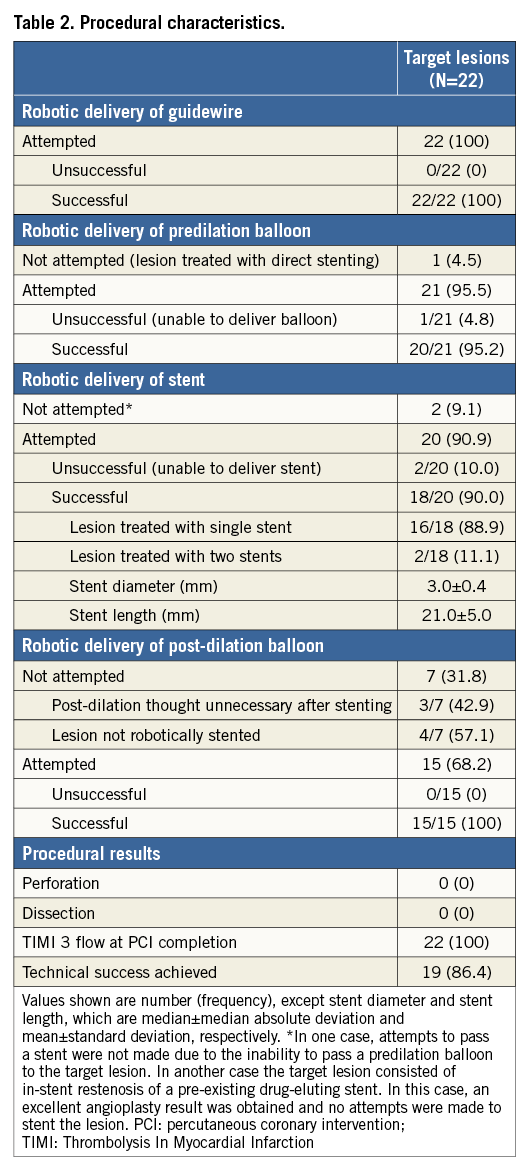
The right radial artery was used for arterial access in all 20 cases. In 18 (90.0%) cases, telecommunications devices provided live audio and visual communications between the two locations throughout the duration of the procedure (Figure 1). In two (10.0%) cases, telestenting was performed using audio telecommunication only, as live video feeds were unavailable. A summary of the interventional approach to all 20 patients is provided in Table 2.
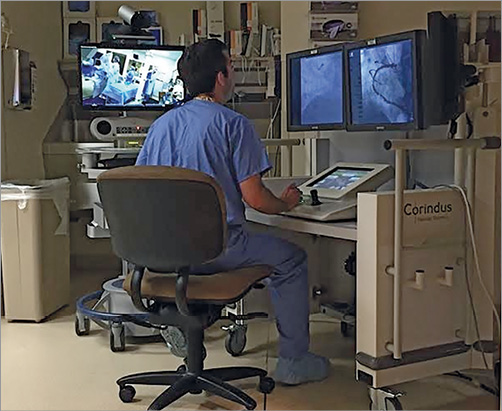
Figure 1. Performance of PCI by a physician operator outside the confines of the procedure room housing the patient. The operator is seated within the robotic cockpit located in an isolated room. Two monitors display real-time fluoroscopy and cineangiography images. A third monitor displays real-time haemodynamic and electrocardiographic data. To the left of the operator is a telecommunications device providing real-time video and audio connectivity to the procedure room housing the patient. PCI: percutaneous coronary intervention
STUDY OUTCOMES
Total procedural time was 29±19 minutes. Total fluoroscopy time was 15.5±7.6 minutes and did not differ significantly in the propensity-matched analysis from the fluoroscopy time of the control cases (15.5±7.6 minutes vs. 18.5±7.7 minutes, p-value 0.22). In the propensity-matched analysis, total air kerma was lower among patients undergoing telestenting compared to controls (1,263±751 mGy vs. 1,826±637 mGy, p=0.01).
Overall technical success was achieved in the treatment of 19 of 22 target lesions (86.4%). Several examples of target lesions successfully treated are depicted in Figure 2. In the three cases where technical success was not met, each required conversion to a manual procedure (Figure 3). In two of the unsuccessful cases, conversion to a manual procedure was required for inability to deliver a stent robotically to the target lesion after successful robotic angioplasty. In one of these two cases, manual delivery of the stent to the target lesion was successful, but required use of a stiff supportive wire (Mailman™; Boston Scientific, Marlborough, MA, USA) in combination with a “buddy wire” technique. The second case was also successful using a manual approach but required selective deep intubation of a guide catheter extension (GuideLiner®; Vascular Solutions, Minneapolis, MN, USA) into the coronary artery to deliver the stent. In the third case requiring conversion to a manual procedure, the target lesion was successfully wired robotically, but the operator was unable to pass a balloon robotically across the target lesion. In this case, attempts to pass a balloon across the lesion using a manual approach were also unsuccessful. This patient was brought back to the catheterisation laboratory approximately one month later and required rotational atherectomy to facilitate stent passage. Thus, overall procedural success was achieved in 19 of 20 patients (95.0%) and did not differ from the procedural success rate of controls (95%) in the propensity-matched analysis. There were no deaths and no instances of repeat revascularisation in any of the patients prior to hospital discharge.
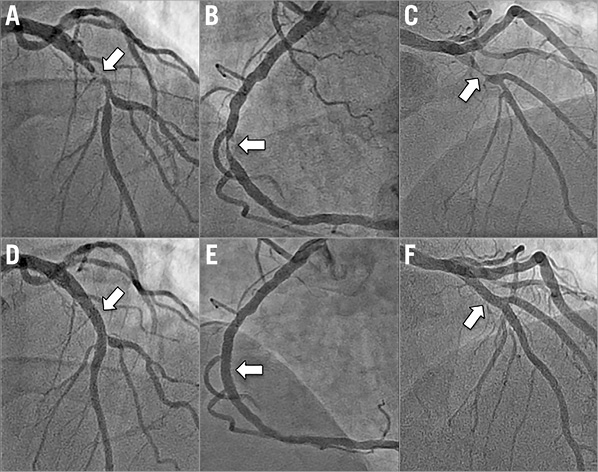
Figure 2. Target lesions in three patients with myocardial infarction successfully treated by an operating physician in a separate physical location from the patient. A) - C) Angiograms of target lesions (arrows) in three patients presenting with myocardial infarction. In each case, the target lesion was successfully treated using telestenting. D) - F) The final angiograms for these cases.
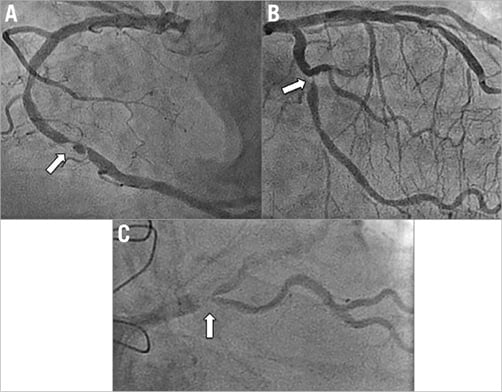
Figure 3. Cases not achieving technical success. Angiograms of three cases in which technical success was not achieved with telestenting are shown. Arrows indicate the target lesions. A) & B) Conversion to a manual procedure resulted in successful stenting. C) The patient was brought back for rotational atherectomy and stenting one month later.
Discussion
To the best of our knowledge, the present study is the first to demonstrate that it is feasible for a physician to perform PCI from outside the confines of the procedure room by using a combination of robotics and telecommunications. Accordingly, PCI was performed successfully in this manner, without death or need for repeat revascularisation prior to hospital discharge, in 19 of 22 target lesions. Albeit limited by inclusion of only a small cohort of patients and the performance of telestenting over a distance of only 55 feet, the present study demonstrates that, using current technology, the operating physician and patient, who were in complete isolation from each other in this study except for the audio and visual connectivity provided by telecommunications devices, are no longer required to be in the same location for stenting to occur. In this regard, the present study represents an early exploration into the feasibility of telestenting, wherein a physician operator uses a combination of robotics and telecommunications to perform PCI on a patient from a separate physical location. Additional studies will determine if further advancements in catheterisation laboratory robotics, namely the development of a wireless connection between the robotic arm and robotic controls, will facilitate the successful performance of telestenting over longer geographic distances.
TELEMEDICINE, TELESURGERY, AND TELESTENTING
The concept of telestenting is an extension of telemedicine, in which medical care is provided remotely using telecommunications7, and telesurgery, in which remote surgery is performed using both telecommunications and robotics8. The adoption of telemedicine has been expanding owing to technological advancements, evident in the results of a 2012 survey in which 42% of acute care hospitals in the USA reported having telemedicine capabilities9. Telemedicine, which has the ability to disseminate advanced medical expertise rapidly to rural hospitals, has been studied in acute stroke and shown to be associated with more accurate medical decision making compared to standard care10.
Advancements in technology have similarly enabled the advent of telesurgery. In fact, several notable non-cardiac telesurgical procedures have been performed to date, including the “Lindberg operation” in September 2001, in which a surgeon located in New York performed a robotic laparoscopic cholecystectomy on a patient in Strasbourg, France, over a distance of some 7,000 km11. Subsequently, a telesurgical service was established in 2005 between two hospitals in Canada that allowed the performance of multiple robotic operations on patients located at a small community hospital by a surgeon at a teaching hospital roughly 400 km away12. Telesurgery is being pursued for other applications as well, as shown in a recent study in which remote robotics were successfully used by an operator in Kagawa, Japan, to perform cerebral angiography on animals located in Beijing, China13. Although it remains unknown if telestenting could be safely performed in remote locations without surgical back-up, extensive data support the safety of performing both elective and primary PCI manually without on-site surgery14.
ROBOTIC PCI
In the Percutaneous Robotically-Enhanced Coronary Intervention (PRECISE) study, which was the first to evaluate the outcomes of robotic stenting, robotic PCI was performed with technical and procedural success rates of 98.8% and 97.6%, respectively4. Furthermore, robotic PCI was associated with a 95% reduction in radiation exposure to the operator4. This dramatic reduction in radiation exposure represents a considerable advantage in favour of robotic PCI compared to manual PCI, especially considering the occupational hazards associated with radiation exposure to interventional cardiologists15,16.
The principal difference between the PRECISE study and the present study is that procedures in PRECISE were performed using robotic controls located in the same room as the patient4. Consequently, effective communication between the operating physician and catheterisation laboratory staff members was not reliant upon telecommunications devices. The observations of the present study extend those of PRECISE by demonstrating the successful performance of robotic PCI when the robotic controls are no longer at the bedside. With the operating physician and patient in separate physical locations, telecommunications devices provided two-way real-time audio and in most cases video connectivity between the two locations, thereby allowing the operating physician to be “virtually present” in the catheterisation laboratory. This “virtual presence” facilitated the necessary communications between the operating physician and catheterisation laboratory staff that are essential during PCI procedures.
POTENTIAL APPLICATIONS OF TELESTENTING
If developed further, telestenting has several potential future applications. By allowing the operating physician to perform PCI outside the room containing the radiation source, telestenting could be applied over short distances, similar to those of the present study, to eliminate operator radiation exposure completely. In the present study, this approach was not associated with any apparent increase in patient radiation exposure compared to a propensity-matched group of controls. However, given the small number of patients in this analysis, additional studies are required to delineate more fully the radiation associated with telestenting procedures.
When performed over longer distances, telestenting has the potential to increase access to PCI in geographic regions where PCI is currently unavailable. In this regard, the role of telestenting in primary PCI could be explored, as STEMI patients frequently present to hospitals lacking PCI capability and require emergent transfer to PCI centres17. In the USA, the transfer of STEMI patients from one hospital to another is rarely associated with door-to-balloon times of <90 minutes or even <120 minutes17. Such delays in STEMI treatment are associated with both greater infarct size and increased mortality18. Considering that primary PCI is associated with lower rates of reinfarction, stroke, and death compared to fibrinolysis, primary PCI via telestenting might also represent a favourable alternative to lytic therapy19. Considering that the robotic system takes only minutes to set up prior to performing robotic PCI, temporal limitations in procedure preparation are unlikely to represent a major hurdle in the development of telestenting for primary PCI.
There are several challenges to address in the implementation of telestenting over long geographic distances. In the present study, the interventional operator obtained arterial access, performed diagnostic angiography, and seated the guide catheter prior to performing telestenting. In geographically remote telestent procedures, a second physician or physician assistant capable of performing such tasks at the bedside would be required. This individual could also be trained to provide technical aid in the event that robotics failed. It is conceivable that these bedside procedural steps might be most readily accomplished by a non-interventional cardiologist experienced in performing coronary angiography but who is not trained in PCI. Such cardiologists possess the skills to engage and manipulate the guide catheter, perform contrast injections, move the operating table and camera, control the fluoroscopy and cineangiography pedals, and manage the vascular access site after stenting.
Study limitations
There are several important limitations to consider when interpreting the results of the present study. First, the present study was limited by its small sample size and single-centre design. Although this study successfully demonstrated the feasibility of performing PCI from outside the confines of the procedure room, larger studies are required to delineate fully the technical and procedural successes of this approach. Second, stenting in the present study was performed over a distance of only 55 feet. This distance, which is currently limited by the length of the cord connecting the robotic controls to the bedside robotic arm, must be increased to make telestenting clinically applicable. This limitation will probably be overcome in the future with the development of a wireless connection between the robotic controls and the robotic arm. Third, the learning curve associated with performing robotic PCI has not been extensively evaluated. In the only published study to date assessing this learning curve, the first three robotic cases performed by new operators were associated with longer procedure durations and fluoroscopy times compared to robotic cases performed with greater experience20. Fourth, initial engagement and subsequent manipulations of the guide catheter are not possible with the currently available robotic device. This lack of guide catheter control represents a greater limitation in telestenting than in bedside robotic PCI and may be overcome with future robotic designs. Fifth, robotic delivery of guidewires, balloons and stents does not provide the operator with tactile feedback characteristic of manual PCI. Despite this limitation, the current-generation robotic system performs well in the delivery of guidewires, balloons and stents4. Finally, technical and procedural success was not achieved in all patients, owing to an inability to pass either an angioplasty balloon or stent across the lesion robotically. Additional studies are required to determine the optimal selection of patients and lesions best suited to undergo telestenting.
Conclusions
To the best of our knowledge, the present study is the first to explore the feasibility of telestenting, wherein a physician operator uses a combination of robotics and telecommunications to perform PCI on a patient from a separate physical location. Additional studies are required to determine if future advancements in robotics will facilitate telestenting over longer geographic distances.
| Impact on daily practice This study explores the feasibility of telestenting and demonstrates that the operating physician and patient, who were in complete isolation from each other during the procedure except for the audio and visual connectivity provided by telecommunications devices, are no longer required to be in the same location for stenting to occur. Additional studies will determine if future advancements in robotics will facilitate the successful performance of telestenting over longer geographic distances. If developed into a procedure capable of being performed over long distances, telestenting could be applied to improve access to stenting in medically underserved regions and might represent a novel alternative to inter-hospital transfer for primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. |
Funding
This work was supported by the Frederik Meijer Heart & Vascular Institute, Spectrum Health, Grand Rapids, MI, USA.
Conflict of interest statement
R. Madder has received research support from Corindus Vascular Robotics, Inc. The other authors have no conflicts of interest to declare.

