
Abstract
Aims: Vascular complications are among the most commonly observed complications after TAVI. Iliofemoral vascular outcome has been described extensively. Little is known about vascular complications in transaxillary TAVI. The aim of the current study was to describe the incidence and predictors of axillary artery complications incorporating computed tomography angiography (CTA) measurements.
Methods and results: CT analysis was performed in two hundred patients treated with transaxillary TAVI in our centre between January 2014 and December 2017. Vascular complications occurred in 37 (18.5%) patients. Patient characteristics predicting this outcome were female gender (aOR 3.88 [1.48-10.14], p=0.006) and age (aOR 1.08 [1.01-1.16], p=0.034). The CTA measurement predicting vascular complications was a sheath to artery area ratio (SAAR) equal to or larger than 1.63 (OR 3.95 [1.29-12.12], p=0.016).
Conclusions: The present study describes the incidence of axillary artery complications and identifies patient characteristics associated with this outcome. CTA analysis was shown to be an important screening tool in the assessment of patient (access) eligibility. Axillary artery dimensional screening should be based on vascular luminal area assessment rather than diameter measurement alone.
Introduction
Transcatheter aortic valve implantation (TAVI) for the treatment of symptomatic severe aortic valve stenosis is currently a well-established treatment in a selected group of patients1. Techniques are still improving, and a worldwide trend is observed towards more minimalistic and less invasive procedures. Despite the tremendous improvements in devices and techniques, vascular complications are among the most frequently observed complications in TAVI2. They consist of access-site or access-related vascular injury (dissection, stenosis, perforation, rupture), distal embolisation or any unplanned endovascular stenting or surgical intervention3 and have been associated with morbidity, prolonged hospital stay, mortality and higher healthcare costs4,5,6.
The femoral artery has been adopted as the primary access in TAVI and results have been described extensively. Incidences of femoral artery complications vary between 1.9 and 33.0%4,5,7,8,9. The left axillary artery (LAA) is often used as an alternative approach when a transfemoral approach is not feasible. In general, the subclavian and axillary artery are less affected by atherosclerosis as compared to the femoral and iliac arteries10,11. Moreover, the modest tortuosity and relative proximity to the native aortic valve render it a good and safe approach for TAVI. However, hardly any reports have been published on the incidence of vascular complications in transaxillary TAVI. Also, it is not known whether computed tomography angiography (CTA)-derived vascular characteristics can predict the occurrence of these vascular complications.
Therefore, the aim of this study was to describe the incidence of vascular complications in transaxillary TAVI. Secondly, we determined predictors of vascular complications based on patient, procedural and CTA characteristics.
Methods
All consecutive patients treated with transaxillary TAVI in the Radboud University Medical Center between January 2014 and December 2017 were included. Treatment allocation was performed by a dedicated Heart Team. At the beginning of our TAVI programme in 2008, the left axillary artery was selected as primary access site in the majority of patients. Axillary access was deemed ineligible in cases where patients had a patent left internal mammary artery (LIMA) bypass graft. Since mid 2016, this strategy changed towards a “femoral first” strategy. As for valve types implanted, CoreValve® and Evolut™ R (Medtronic, Minneapolis, MN, USA) were predominantly used. All procedures were performed under general anaesthesia and axillary artery access and closure were performed surgically. Additional procedural details and results have been published previously12,13. Patients were excluded from current analysis in cases where CTA (1) was not performed/available or (2) was of poor quality (insufficient luminal contrast/blurring/artefacts) or did not visualise the left subclavian/axillary artery (LSAA) from the aorta to the origin of the lateral thoracic artery. The current study was approved by the institution’s ethics committee and complies with the Declaration of Helsinki.
The primary outcome of the present study was the occurrence of a vascular complication consisting of dissection, stenosis and perforation or rupture of the LSAA. After TAVI, the LSAA was assessed for adverse vascular outcome by means of conventional angiography, performed by an experienced interventional cardiologist. Endovascular stenting was performed in case of severe flow-limiting stenosis (Supplementary Figure 1). LSAA perforation or rupture was treated with covered stents. For this study, all angiograms were analysed again by a second investigator (K. van der Wulp). In case of any doubt, cases were discussed with an experienced interventional cardiologist (N. van Royen) for final adjudication. Prior to data analysis, all data fields were examined for missing data, improbable values or fields indicating “unknown” and, when indicated, excluded from analysis (less than 1% of all data).
CTA ANALYSIS
Patients underwent a clinically indicated ECG-triggered CTA prior to evaluation for TAVI per standard institutional protocols. The majority of scans were performed in our institution on a 320-slice platform (Aquilion GENESIS; Canon Medical Systems USA, Inc., Tustin, CA, USA) and patients received 70-90 mL of Omnipaque Iomeron 400 (Patheon Italia, Ferentino, Italy). CTA analysis was performed using 3mensio software (version 5.1; Pie Medical Imaging, Maastricht, the Netherlands) on a dedicated three-dimensional (3D) workstation with the ability to manipulate images in a double oblique fashion. For this study, all CTA measurements were performed by two investigators (I. Thijs, K. van der Wulp) who were blinded to the primary outcome. For quantification of luminal dimensions, semi-automatic post-processing using a centreline placement was used. Manual verification of the centreline was performed to ensure accurate vessel tracking and appropriate intraluminal location of the centreline.
The tortuosity index (TI) was calculated by dividing the centreline distance (from the ostium of the left subclavian artery to the origin of the left lateral thoracic artery) by the straight-line distance (measured in the frontal view between the same origins)10. A calculated index greater than 1.0 indicated the existence of curvatures in the LSAA.
Calcification measurements were based on eyeballing and pixel probe density measurement. Three segments of 5 centimetres each were analysed. Per segment, a four-point Likert scale was used to indicate the amount of calcification (none, mild, moderate or severe) followed by calcification pattern (local stenosis, diffuse stenosis or a combination of both), the circumferential amount (0-90°, 90-180°, 180-270° or 270-360°) and Agatston score measured at a calcified location with the lowest minimal luminal diameter.
Minimal luminal diameter (MLD) and minimal luminal area (MLA) were assessed by manual drawing of a line surrounding the contrast-filled lumen in a perpendicular view at the location where the software automatically calculated the smallest diameter. Sheath to artery ratio (SAR) and sheath area to artery area ratio (SAAR) were calculated by dividing the sheath outer diameter (OD) by the MLD for SAR and the sheath area by MLA for SAAR. Calculated ratios >1.0 indicate a sheath size that exceeds the MLD or MLA of the artery (Figure 1).
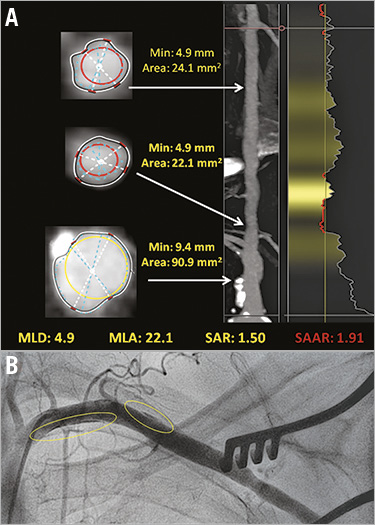
Figure 1. Subclavian/axillary artery CTA measurements and dissection. Illustration of CTA measurements of LSAA with derived ratios (A) and angiography after TAVI showing two dissections of the artery (B, circled).
STATISTICAL ANALYSIS
Categorical variables were presented as percentages and frequencies and compared using the chi-square or Fisher’s exact test. Continuous variables were expressed as means±standard deviations (SD) if normally distributed or as median (interquartile range [IQR]) if skewed and compared using the Student’s t-test or Mann-Whitney U test, respectively.
Univariate associated baseline, procedural and CTA factors (with a p-value <0.05) were entered into a multivariable logistic regression model and predictors were selected using a backward elimination procedure. Odds ratios (OR) and corresponding 95% confidence intervals (95% CI) were computed. Collinearity diagnostics were evaluated for all variables considered for multivariable analysis. In case of multicollinearity, the variable with the higher odds ratio (OR) was incorporated into the model. Optimal cut-offs were assessed using Youden’s index of receiver operating characteristic (ROC) analysis.
All tests were two-tailed, and a p-value <0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics software, Version 25.0.0.1 (IBM Corp., Armonk, NY, USA) and illustrated with GraphPad Prism, version 5.03 (GraphPad Software Inc., San Diego, CA, USA).
Results
Between January 2014 and December 2017, a total of 469 patients were treated with TAVI in our institution. Axillary access was used in 305 patients. One hundred and five (105) patients had to be excluded due to either non-availability of CTA (n=63, because conventional angiography was performed to assess axillary eligibility) or poor CTA quality or incomplete visualisation of the LSAA (n=42) (Supplementary Table 1). CTA analysis was completed in 200 patients. In these patients, procedural success was achieved in 93.5% (n=187) (Supplementary Table 2).
Vascular complications of the LSAA were observed in 37 (18.5%) patients, consisting of one major vascular complication and 36 minor vascular complications (Supplementary Table 2). The major vascular complication concerned a patient who died two days after TAVI. Autopsy of this patient revealed a rupture of the ostium of the left subclavian artery. Minor vascular complications were predominantly located at the access site (56.8%), consisting of dissection (n=32, 16%), stenosis (n=3, 1.5%) and perforation or rupture (n=2, 1.0%). Unplanned endovascular stenting or surgical intervention was performed in 14 (7.0%) and three cases (1.5%), respectively. Non-flow-limiting stenoses were left untreated. One patient (0.5%) had in-stent thrombosis in the axillary artery, 93 days after TAVI, which was successfully stented. None of the other patients had complaints of hand claudication or loss of function during follow-up. In-hospital death occurred in six (3.0%) patients one of which was related to a vascular complication (as described above). After discharge, 18 (9.0%) patients died within one year. None of these patients had experienced a TAVI-related vascular complication.
Baseline characteristics are described in Table 1. Patients with an observed LSAA complication were older (82 [79-85] vs 80 [75-83], p=0.010), more often female (83.8% vs 49.7%, p<0.001) and had a lower body surface area (1.78 [1.69-1.85] m2 vs 1.90 [1.73-2.03] m2, p<0.001) and smaller aortic valve area (0.70 [0.50-0.80] cm2 vs 0.80 [0.66-0.90] cm2, p=0.010). Although not significant, arterial calcification in the form of peripheral artery disease was observed more often in patients with vascular complications (37.8% vs 27.0%, p=0.189).
Regarding the procedure, SoloPath™ inflatable sheaths (Terumo Corp., Tokyo, Japan) with an outer diameter of 7.0-7.5 mm were most often used (78.0%) and did not differ between the groups (Supplementary Table 2). Procedural treatment actions/manipulation of the LSAA (e.g., predilatation or post-dilatation, resheathing, retrieval or second device implantation) were not performed more often in patients with vascular complications. In case of a vascular complication, both procedural and hospital admission duration were significantly longer (61 [50-84] min vs 51 [39-63] min, p=0.004, and 6 [4-8] days vs 4 [3-7] days, p=0.011, respectively).
CTA measurements (Table 2) showed an overall median length of the LSAA of 17.62 (16.16-18.89) centimetres. Corrected for body surface area (BSA), no significant difference was observed in total artery length between patients with or without vascular complications (p=0.645). The amount of tortuosity, expressed by means of the TI also did not differ between the groups (1.43 [1.32-1.55] vs 1.40 [1.32-1.50], p=0.480).
Calcification was most often observed in the proximal 5 centimetres of the subclavian artery (in subsequent segments of 5 cm, from proximal to distal: 81.5%, 29.5% and 11%) and was classified as moderate or severe in 16.5% (33) of the patients. This prevalence did not significantly differ between patients with or without vascular complications (p=0.661). The circumferential amount of calcification most often did not exceed 90° (n=130, 65%). More circumferential calcified arteries (>90°) tended to be observed in patients with vascular complications (48.6% vs 31.9%, p=0.054).
MLD in the first 15 cm of the LSAA was 4.40 mm and was not significantly different between patients with or without vascular complications (4.10 [3.75-4.65] mm vs 4.40 [3.90-5.00] mm, p=0.108). Also, the SAR was not different between the groups (1.75 [1.58-1.91] vs 1.63 [1.47-1.88], p=0.116). However, the MLA was significantly smaller in patients with vascular complications (19.90 [16.65-24.45] mm2 vs 24.50 [19.60-29.40] mm2, p<0.001) and the SAAR was significantly higher (2.11 [1.74-2.43] vs 1.72 [1.43-2.10], p<0.001).
Univariate logistic regression analysis was performed for baseline and procedural characteristics as well as for CTA measurements. Multivariable logistic regression analysis showed that, for baseline characteristics, female gender (adjusted odds ratio [aOR]: 3.88; 95% CI: 1.48-10.14, p=0.006) and age (aOR: 1.08; 95% CI: 1.01-1.16, p=0.034) were independent predictors of vascular complications (Table 3, Supplementary Table 3). Of the CTA measurements, corrected for the several baseline and procedural characteristics, SAAR with a cut-off of ≥1.63 (Supplementary Figure 2) was the strongest independent predictor of vascular complications (aOR: 3.95; 95% CI: 1.29-12.12, p=0.016).
Discussion
Vascular complications after transaxillary access are frequently observed and associated with elderly age, female gender and valvular calcification. Exceeding a sheath area to vessel lumen area ratio of 1.63 results in four times greater odds of vascular complications (Figure 1).
LSAA complications were observed in 18.5% of patients and consisted mainly of arterial dissections. Previous publications on vascular complications in transaxillary TAVI reported incidences varying between 6 and 11% but comprised studies with smaller patient populations which did not focus primarily on vascular complications14,15,16. Compared to vascular complication incidences reported in transfemoral TAVI (1.9-33.0%)4,5,7,8,9, our observed incidence is relatively high. It is unclear whether there are specific vascular properties which render the axillary artery more prone to arterial dissection. Also, routinely performed LSAA angiography was used to rule out vascular complications. Since this is not standard in transfemoral TAVI procedures, this could potentially explain our findings. Another explanation could be found in the overall smaller diameter as compared to that of the femoral artery10.
Patient characteristics elderly age, female gender and lower body surface area were found to be associated with vascular complications. These findings are in line with previous publications on vascular outcome after transfemoral TAVI5,7,8. We know that ageing causes increased arterial stiffness with endothelial dysfunction and that the presence of cardiovascular disease and chronic inflammation/stressors accelerate this process and can result in a highly vulnerable vasculature17. Furthermore, female gender is a known risk factor, probably due to the lowering/absent oestrogen levels, smaller body surface areas and inherent smaller vessel diameters in females7,9,18.
The present study is the first to describe CT angiographic properties of the LSAA in a large series of transaxillary TAVI patients in relation to vascular complications. Arnett and colleagues demonstrated the relatively small calibre and low atherosclerotic burden of the LSAA as compared to that of the lower extremity vessels10. Additional to their findings, we found that vascular complications occur predominantly at the access site and rarely lead to major adverse outcome. Furthermore, our data show that CTA analysis can serve as an important screening tool in the assessment of access eligibility.
Regarding luminal dimensions, diameter was not associated with vascular outcome. This is in contrast to the current daily practice and literature in which transaxillary TAVI is discouraged in case of an artery diameter of less than 6 mm (or even 7.5 mm in patients with a patent LIMA)19. Furthermore, sheath outer diameter or sheath to artery diameter ratios were also not associated with vascular complications. This is in line with previous findings on sheath sizes and vascular complications20 and underlines the need for other dimensional parameters. MLD assessment does not correct for oval deformation by outer compression of vessels and should therefore be interpreted with caution.
MLA was significantly smaller in patients with vascular complications, and its derived SAAR was the strongest independent CTA measurement predicting vascular complications in our study. An optimal cut-off of 1.63 was determined. Krishnaswamy and colleagues determined a lower cut-off of 1.35 for the prediction of iliofemoral vascular complications8. Besides presumable different vascular properties with less calcification in the axillary artery, it is also possible that differences in CTA assessment and/or sheaths could have caused these different cut-off values. For clinical purposes, based on our findings, we estimated the minimal LSAA diameter and area required for different sheaths (Table 4). These should be interpreted with caution and incorporated in the screening assessment of a patient’s candidacy for (transaxillary) TAVI procedures to minimise further the risk of adverse vascular outcome.
Limitations
This study has some limitations. It is a retrospective, non-randomised single-centre study with a sample size and number of events which limited the number of adjusting variables in the regression analysis. Exclusion of patients may have led to selection bias. Throughout the study period, resheathable devices were more often used, and resheathing was more often performed, possibly influencing the occurrence of vascular complications (Supplementary Table 4). The SoloPath inflatable sheath whose inflatable quality provides easy introduction in relatively small arteries was predominantly used. However, theoretically, it could also have caused plaques or calcifications to be pushed against the arterial wall, leading to higher risks of vascular complications. Differences in CTA acquisition and assessment may lead to alternative findings in future studies.
Conclusions
The present study described the incidence of LSAA complications and identified patient characteristics associated with this outcome. CTA analysis was shown to be an important screening tool in the assessment of patient (access) eligibility. Axillary artery screening should focus on vascular luminal area assessment instead of diameters only.
|
Impact on daily practice The current study is the first study to describe LSAA complications in detail in a large patient cohort. It identified patient characteristics associated with this outcome and incorporated the use of diameter- and area-based CTA measurements. The sheath to artery area ratio was identified as an important predictor of vascular complications. Based on this study, clinicians should incorporate this ratio in their screening assessment of a patient’s candidacy for (transaxillary) TAVI procedures to minimise further the risk of adverse clinical outcome. |
Acknowledgements
The authors gratefully acknowledge the efforts of Carlijn Verkroost with regard to her critical review of the manuscript and Geert Versteeg for his commitment regarding data management.
Funding
The Departments of Cardiology and Cardiothoracic Surgery at Radboud University Medical Center, with which the authors are affiliated, have received an unrestricted grant from Medtronic.
Conflict of interest statement
M. van Wely has been a proctor and consultant for Abbott Vascular. M. Verkroost has been a proctor for Medtronic. H. Gehlmann has been a proctor for Abbott Vascular and Medtronic. L. Van Garsse has been a proctor for Edwards Lifesciences. W. Morshuis has been a consultant for Vascutek. N. van Royen has been a consultant for Medtronic and Amgen and has received institutional grants from AstraZeneca, Biotronik, Philips and Abbott as well as speaker fees from Abbott. All other authors have no relationships relevant to the contents of this paper to disclose.

