
Abstract
Aims: The aim of this study was to evaluate, in anaemic patients, the efficacy of erythropoietin (EPO) in reducing red cell (RC) transfusion rates post TAVI.
Methods and results: This was a randomised double-blind trial. Patients with severe symptomatic aortic stenosis and concomitant anaemia with an indication for TAVI were randomised (1:1) to receive two weight-based doses of EPO (darbepoetin alfa)+iron or placebo at days 10 (±4 days) and 1 (±1 day) pre TAVI. The primary outcome was the rate of RC transfusions at 30 days. A total of 100 patients (mean age 81±7 years, male 49%) were included: 48 patients received EPO (+iron) and 52 patients received placebo. Baseline characteristics and procedural findings were well balanced between groups except for baseline haemoglobin levels, which were lower in those patients receiving EPO (10.7±1.2 vs. 11.3±1.1 g/dl, p=0.01). The rate of 30-day RC transfusion was similar in both groups (27.1 vs. 25.0% in the EPO and placebo groups, respectively; adjusted odds ratio 1.05, 95% CI: 0.42-2.64, p=0.92), and no differences were observed in the number of RC units per transfused patient (1 [1-3] vs. 2 [1-2] in the EPO and placebo groups, respectively, adjusted p=0.99). Rates of 30-day mortality, stroke, new-onset atrial fibrillation, acute kidney injury, and troponin peak were also similar between groups (p>0.20 for all).
Conclusions: EPO (+iron) administration failed to reduce RC transfusion rates or the per-patient number of transfusion units in anaemic patients undergoing TAVI. ClinicalTrials.gov Identifier: NCT02390102
Abbreviations
AF: atrial fibrillation
EPO: erythropoietin
RC: red cell
TA: transapical
TAO: transaortic
TAVI: transcatheter aortic valve implantation
TF: transfemoral
Introduction
Transfusing allogenic red blood cells is associated with increased morbidity and mortality following cardiac interventions1-4. A heightened risk of infectious complications, ischaemic events, acute renal injury and early and late mortality has been observed in patients receiving red cell (RC) transfusions after cardiac surgery1,2,5, and it has been suggested that avoiding RC transfusions may decrease costs by 40%1. Interventions to reduce transfusions have therefore become a priority in cardiac surgery, particularly in patients at high risk for RC transfusions such as those with advanced age and/or preoperative anaemia6. The use of preoperative erythropoietin (EPO) to stimulate red blood cell production is a strategy currently recommended in this setting6. Randomised studies and meta-analyses have shown that administering EPO, in combination with iron, prior to cardiac surgery in non-anaemic patients is associated with an increase in preoperative haemoglobin levels and a reduction in perioperative RC transfusions7-17. A few studies have also suggested a protective effect in anaemic patients, with a reduction >50% in the need for RC transfusions in those patients receiving EPO8,9,15,18.
A high proportion of patients undergoing transcatheter aortic valve implantation (TAVI) require RC transfusions4,19, mostly due to procedural blood loss in combination with preoperative anaemia4,20, which occurs in approximately 60% of patients4,19,21. Similarly to cardiac surgery, the use of RC transfusions in patients undergoing TAVI has been associated with an increased risk of early and late mortality4,22. However, no evidence currently exists on the effects of EPO in the context of TAVI. In the EPICURE trial, we evaluated the efficacy of EPO for reducing the rate of RC transfusions in anaemic patients with severe symptomatic aortic stenosis undergoing TAVI.
Methods
STUDY DESIGN AND OVERSIGHT
EPICURE was an investigator-initiated, randomised, double-blind, placebo-controlled trial. Patients were assigned to receive EPO (+iron) or placebo using a computer-generated randomisation schema in a 1:1 ratio, stratified by planned route (transfemoral [TF] vs. transapical [TA]/transaortic [TAO]). Randomisation was performed by a technician who was not otherwise involved in the study. No patient withdrawals occurred between the time of randomisation and treatment administration. Treatment assignments were concealed from the investigators gathering data and assessing outcome and safety events until the study was complete. All data were prospectively collected. Data regarding RC transfusions were further confirmed using the data from the local blood bank (Héma-Québec, Canada), which was blinded regarding treatment groups. Patients were followed for 30 days and no patients were lost at follow-up. The study investigators designed the protocol, enrolled the patients, analysed the data and wrote the manuscript. All authors vouch for the accuracy and completeness of the data and analyses. The study met the requirements of the Declaration of Helsinki and was approved by the local ethics committee. Written informed consent was obtained from all patients.
Patients eligible for enrolment were ≥60 years old with severe aortic stenosis and anaemia and had an indication for TAVI. Anaemia was defined according to the World Health Organization definition as a haemoglobin value <13.0 g/dl in men or <12.0 g/dl in women in at least one blood sample obtained within the three months prior to TAVI. The main exclusion criteria included any treatment with EPO within a 30-day period prior to TAVI, anticipated non-response to EPO therapy (such as the presence of anaemia due to bone marrow aplasia, haemoglobinopathies or active bleeding), uncontrolled hypertension defined as a systemic arterial pressure >175/95 mmHg, and a high risk for thromboembolic events.
EPO AND TAVI PROCEDURES
Patients received darbepoetin alfa 0.75 µg per kg (Aranesp®; Amgen Inc., Thousand Oaks, CA, USA) + 200 mg intravenous iron sucrose (Venofer®; American Regent, Inc., Shirley, NY, USA) or placebo (0.9% saline) at days 10 (±4) and 1 (±1) prior to TAVI. The EPO (or placebo) was first administered subcutaneously, followed by an intravenous iron infusion (or placebo). The intravenous tubing used for administering the iron (or placebo) was covered with a drape to guarantee that the patient could not see the infusion solution. Both the EPO (+iron sucrose) and placebo were administered by a nurse not involved in the screening, randomisation or assessing of patient outcomes. The investigator was not present during the infusion to ensure the blindness of treatment allocation.
TAVI procedures were performed according to the standards of care23. Eligibility for TAVI, type of transcatheter heart valve and access route were determined by a local Heart Team composed of interventional cardiologists and cardiac surgeons. The TF route was prioritised as the standard practice. Heparin was administered after access was obtained to achieve an activated coagulation time >250 sec. For TA and TAO procedures, a cell saver was used, and collected and processed blood was reinfused to patients. Protamine sulphate was administered at the end of the procedures. The indication of antithrombotic therapy during the hospitalisation and at hospital discharge was left to the discretion of the responsible physician. In patients receiving clopidogrel, it was initiated the day before TAVI if a TF route was used or 24 hours after the procedure in TA/TAO TAVI.
In accordance with the Society of Thoracic Surgeons and the Society of Cardiovascular Anaesthesiologists Blood Conservation Clinical Practice Guidelines, RC transfusions were triggered by a haemoglobin level of 7.0 g/dl or a haematocrit less than 22% during the perioperative period. As per current practice, RC transfusions were also indicated if symptoms of anaemia occurred in patients with a haemoglobin level between 7.0 and 8.0 g/dl, or regardless of the haemoglobin value if a life-threatening bleeding occurred6.
STUDY OUTCOMES
The primary outcome was the rate of RC transfusions within 30 days after TAVI. Secondary outcomes included the number of RC units administered, the haemoglobin concentration within the 24 hours before TAVI and at hospital discharge, the peak of troponin and creatine kinase-MB, the rates of 30-day mortality, myocardial infarction, stroke, and the combined endpoint of RC transfusion, myocardial infarction or stroke, the incidence of acute kidney injury, need for haemodialysis and new-onset atrial fibrillation (AF). Outcomes were defined according to the Valve Academic Research Consortium-2 criteria24.
SAMPLE SIZE CALCULATION
Based on prior data, EPO administration was expected to result in a 50% reduction in RC transfusion rates8,9, and a 60% transfusion rate was anticipated in the placebo group19. A total of 45 patients per group would provide an 80% power to detect differences between groups, with a two-sided significance level of 0.0525.
STATISTICAL ANALYSIS
Categorical variables are expressed as numbers (percentage) and quantitative variables are reported as mean±standard deviation or median (interquartile range). The two-sided chi-square or Fisher’s exact test was used for comparisons of categorical variables, including primary and secondary outcomes. A logistic regression model was used to determine the impact of study treatment on dichotomous outcomes after adjusting by differences in haemoglobin concentration at baseline. Continuous variables were analysed using t-tests or Mann-Whitney U tests, depending on distribution of variables. At multivariate level, quantitative outcomes were analysed using generalised linear models. Generalised linear models were also used to determine risk ratios and p-values for interactions in subgroup analyses. A multivariate zero-inflated Poisson regression model was used to determine the association between study treatment and the number of RC units transfused. Results were adjusted by the haemoglobin concentration before randomisation. Kaplan-Meier estimates and a log-rank test were used to compare time to RC transfusion according to study groups. Changes in haemoglobin concentration from baseline were analysed using a general linear model for repeated measures. Subgroups were defined according to age, sex, the presence or absence of diabetes, chronic kidney disease and severe anaemia defined as a haemoglobin level <100 g/L, and the route used for TAVI.
A p-value <0.05 was considered statistically significant and all tests were two-sided. Statistical analyses were performed using the SAS software, Version 9.3 (SAS Institute Inc., Cary, NC, USA) and the Statistical Package for Social Sciences, Version 20 (IBM Corp., Armonk, NY, USA).
Results
PATIENTS AND STUDY PROCEDURES
From a total of 307 TAVI candidates, 188 (63.1%) had anaemia before the procedure. Among them, 104 patients were finally enrolled and received the study treatment: 51 patients were assigned to darbepoetin alfa (+iron) and 53 patients were assigned to placebo. Two patients receiving EPO and one patient receiving placebo infusions did not finally undergo TAVI and were excluded from the analyses. One additional patient was excluded due to active bleeding before TAVI. Therefore, the final study population consisted of 100 patients, 48 patients in the EPO (+iron) group and 52 patients in the placebo group. All patients received the complete dose of the assigned therapy except for one patient included in the EPO group who refused the second dose of treatment (Figure 1).
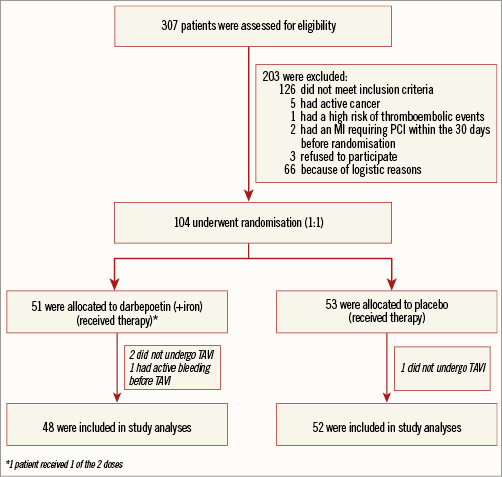
Figure 1. Flow chart of the EPICURE study population. Out of a total of 307 patients screened, 104 patients underwent randomisation and 100 patients were finally included in study analyses. MI: myocardial infarction; PCI: percutaneous coronary intervention; TAVI: transcatheter aortic valve implantation
The mean age of the study population was 81±7 years, 49 (49.0%) patients were male and the mean haemoglobin value at inclusion was 11.2±1.1 g/dl in men and 10.5±9.0 g/dl in women. Baseline clinical and echocardiographic findings are shown in Table 1. The two groups were well balanced except for the haemoglobin concentration at randomisation which was lower in the EPO group (10.7±1.2 vs. 11.3±1.1 g/dl, p=0.011).
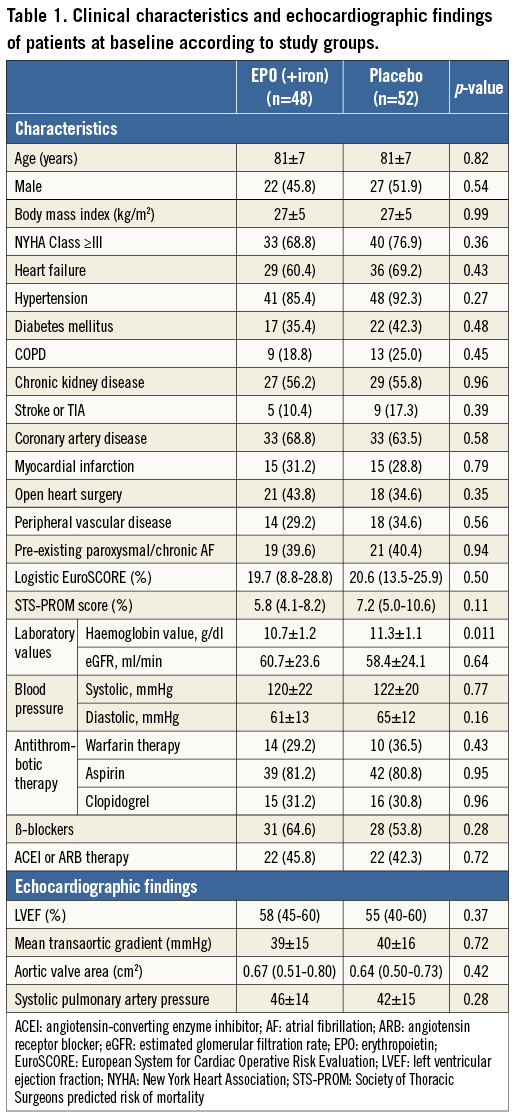
The procedural findings are shown in Table 2. No differences were observed between groups in procedural complications (p>0.20 for all).
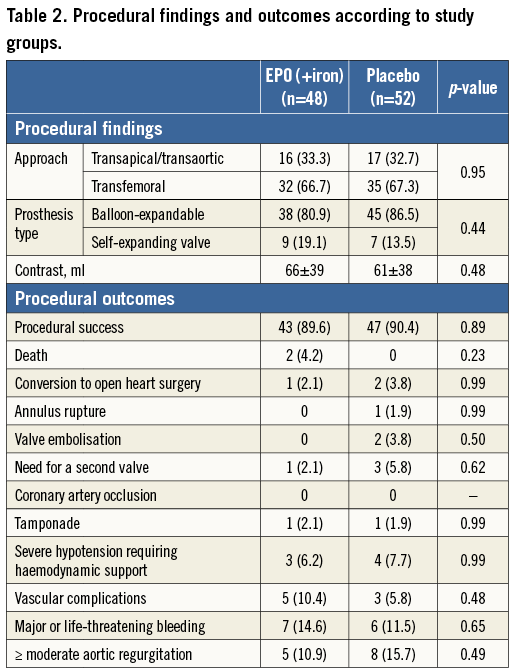
CHANGES IN HAEMOGLOBIN CONCENTRATION AND RC TRANSFUSION
Changes in haemoglobin concentration during the hospitalisation period are shown in Figure 2. Overall, no significant differences were observed between study groups (p=0.19). Patients receiving EPO had a non-significant increase in haemoglobin level of 0.2±0.9 g/dl (from 10.7±1.2 to 10.9±1.2 g/dl, p=0.14) before the TAVI procedure, and patients receiving placebo had a non-significant decrease of 0.2±0.7 g/dl (11.3±1.6 to 11.1±1.2 g/dl, p=0.054), p=0.018 for differences between groups. No differences were observed between study groups in the absolute haemoglobin value before the procedure (10.9±1.2 g/dl in the EPO group vs. 11.1±1.3 g/dl in the placebo group, p=0.39), drop of haemoglobin after TAVI (2.1±1.1 g/dl in the EPO group vs. 2.2±1.1 g/dl in the placebo group, p=0.822) or in the haemoglobin value at hospital discharge (9.4±1.6 g/dl vs. 9.9±1.6 g/dl in the EPO and placebo groups, respectively, p=0.12).
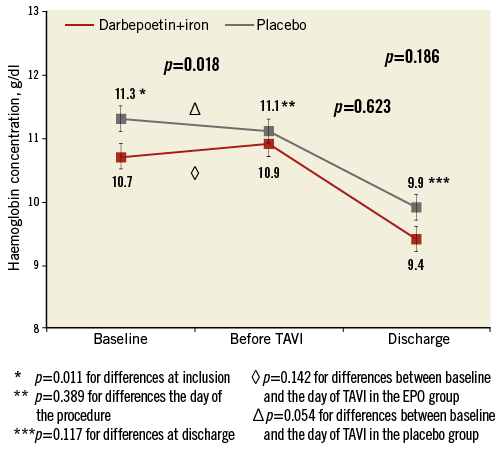
Figure 2. Changes in haemoglobin levels. Changes in haemoglobin concentration from baseline to the day before the procedure and at hospital discharge, according to study groups. Error bars represent mean±standard error of the mean.
PRIMARY AND SECONDARY OUTCOMES
Thirty-day primary and secondary outcomes are shown in Table 3. A total of 26 (26.0%) patients received RC transfusions within the first 30 days after TAVI, 13 (27.1%) patients in the EPO and 13 (25.0%) patients in the placebo group (p=0.81). These results remained similar after adjusting for haemoglobin concentration at randomisation (OR: 1.05, 95% CI: 0.42-2.64, p=0.92). No differences were observed in the median time to RC transfusion among transfused patients between study groups: 2 (0.5-4.5) days in the EPO (+iron) vs. 2 (0.5-4.0) days in the placebo group (p=0.92). The RC transfusion rates over time were not different between groups (Figure 3). Overall, 57 RC units were transfused, 26 (45.6%) in the EPO group and 31 (54.4%) in the placebo group. This lack of association between EPO and transfusion persisted when a landmark analysis with a cut-off at 1 day and 7 days (before and after 1 and 7 days) was performed. Among the transfused patients, the median number of RC units was 1 (1-3) in the EPO (+iron) group and 2 (1-2) in the placebo group. A zero-inflated Poisson model confirmed the failure of EPO therapy to reduce the number of RC units (incidence rate ratio: 0.77, 95% CI: 0.27-2.19; p=0.63, adjusted p=0.99). Results were consistent across subgroups of patients with respect to the primary outcome. No significant interactions were observed between treatment and any of the subgroups (Figure 4).
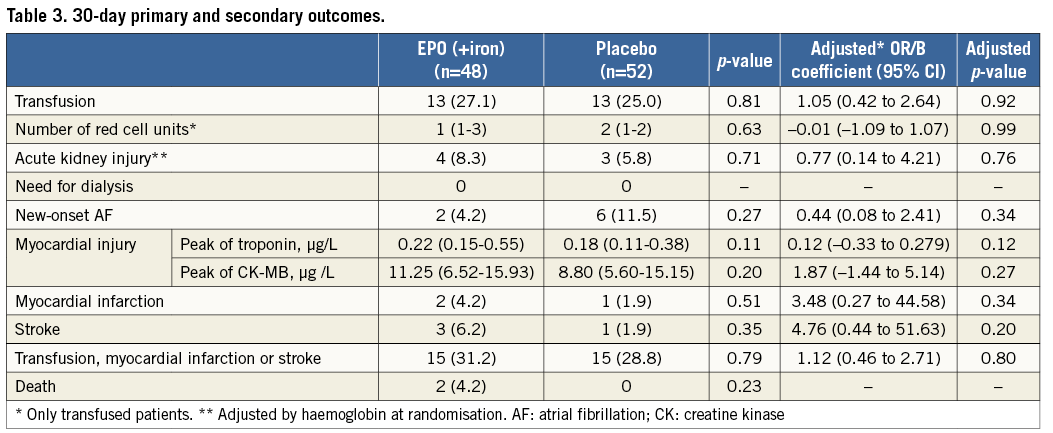
Figure 3. Rates of red cell transfusion. Kaplan-Meier curve for the primary outcome according to study groups.
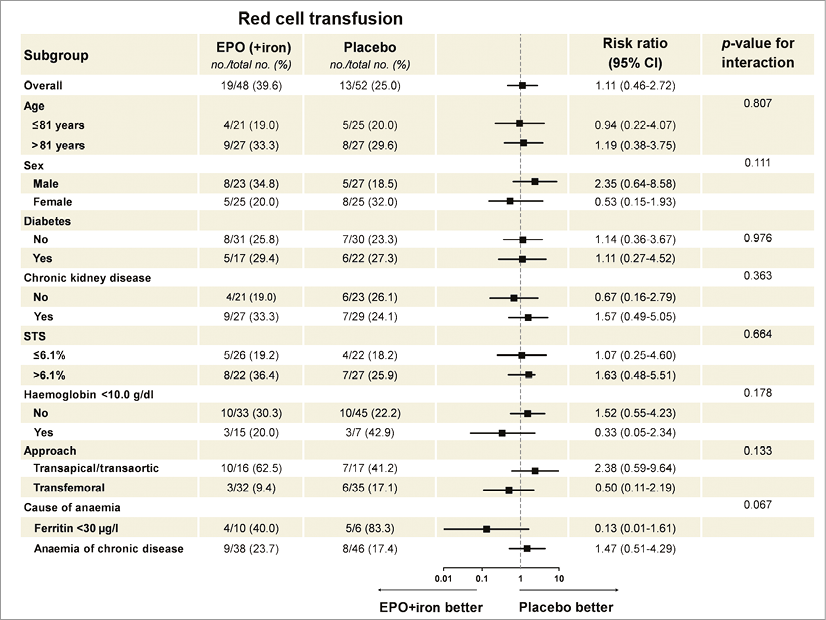
Figure 4. Subgroup analyses of the primary endpoint. The forest plot represents the relative risk and 95% confidence intervals of the primary endpoint according to subgroups.
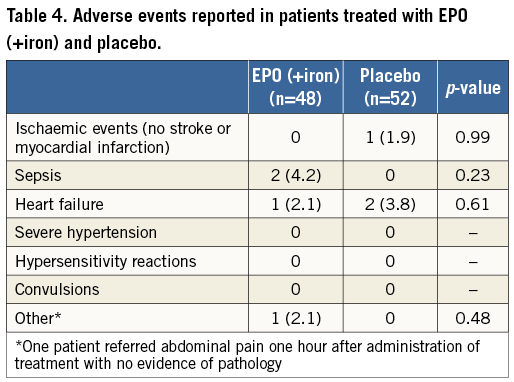
The rates of acute kidney injury, need for dialysis, new-onset AF, myocardial infarction, stroke, and death were not different between groups (adjusted p>0.2 for all). The combined endpoint of RC transfusions, stroke and myocardial infarction occurred similarly in both groups (adjusted p=0.79). The median stay in hospital was 6 (5-12) days in the EPO group and 6 (5-9) in the placebo group (adjusted p=0.73).
All adverse events observed during the study period are displayed in Table 4. Hypertension, convulsion or hypersensitivity reactions did not occur during the study period. No significant differences were observed in the rates of ischaemic events, heart failure, infectious complications or convulsions between groups (p>0.50).
Discussion
These results differ from those of most prior studies in the cardiac surgical field, which demonstrated significant reductions in RC transfusions and number of RC units in patients receiving EPO before the surgical intervention7-17. Also unlike the present study, prior studies showed both an increase in the haemoglobin levels (about >1.5 g/dl) pre-intervention and a higher haemoglobin concentration nadir associated with EPO7,9-11. However, while most prior studies included essentially non-anaemic patients7,9,11-14,16,17, only anaemic patients were included in this study, with up to 22% being severely anaemic. A few randomised studies have evaluated the effect of EPO in anaemic patients undergoing cardiac surgery, suggesting a reduction in the need for RC transfusions and number of RC units in patients receiving this therapy8,9,15. Nonetheless, most prior studies in anaemic patients included limited sample sizes, haemoglobin concentrations pre-intervention which were often >11.5 g/dl (compared with <10.8 g/dl in EPICURE), younger patients (<75 years vs. >80 years in EPICURE), less heart failure (<5% vs. 65% in EPICURE), exclusion of patients with renal impairment and, overall, patients in prior studies harboured a lower risk profile with a lower prevalence of comorbidities.
Exogenous EPO binds to the endogenous EPO receptor, stimulating proliferation and maturation of erythroid progenitor cells into reticulocytes and mature erythrocytes, avoiding apoptosis. An increase in the reticulocyte count may be observed at day three, and it has been reported that the equivalent to one unit of blood is produced by day seven after the administration of EPO26. However, the effect of EPO is reduced by the presence of inflammation which inhibits the growth of erythroid precursor cells, disrupts iron metabolism, induces changes in the EPO receptor and interferes with post-receptor signalling routes27. Indeed, heightened inflammatory status, as occurs in the elderly, chronic kidney diseases and heart failure, reduces the production and response to EPO, serving as the main cause of EPO resistance and one of the main causes of anaemia in such patients27,28. In addition, the use of β-blockers and inhibitors of the renin-angiotensin system, which were frequently used in patients included in this study, have been associated with a reduced effect of EPO27,29.
In addition to inflammation, iron and B-12 vitamin deficiency are predictors of poor response to EPO therapy30,31. Importantly, iron deficiency anaemia occurs in up to 50% of anaemic patients undergoing TAVI21. Although a small dose of intravenous iron was administered in this study, this was not intended to replete iron stores, but to compensate for the decrease in iron indexes associated with accelerated EPO-induced erythropoiesis and to maximise the effect and increase the cost-effectiveness of EPO therapy31.
Other factors may also have contributed to the discrepancies between this study and prior observations. First, the rate of blood transfusions associated with TAVI, in particular when using the TF route, which was the most frequently used TAVI route in this study, is lower than that required in cardiac surgery32,33. In fact, a progressive increase in the use of TF TAVI occurred during the study period. Furthermore, an evolution of the technique with the incorporation of several improvements (i.e., the use of the radial approach for the contralateral as secondary access) was observed over the study period, and this resulted in a reduction in the rate of bleeding complications.
The restriction in blood transfusion policies to follow current recommendations might further explain the lower rate of blood transfusion observed in our study (including the control group) compared with other TAVI studies4,19,21. Second, the strongest beneficial effect of EPO in reducing RC transfusions has been observed in patients included in programmes of autologous blood transfusions, which was not available in this study16. However, the low concentration of haemoglobin in these patients, with a poor tolerance to anaemia due to the presence of aortic stenosis and frequent coronary artery disease, might have precluded their inclusion in such programmes34. Third, although both variables varied widely across studies, the dose and interval between doses of EPO might have played a role in the results of this study. Greater total doses administered during longer periods of time pre-TAVI might have optimised the true effect of EPO. However, this greater effect may be accompanied by an increased rate of thrombotic events. This is particularly important in patients with EPO resistance, in whom the risk of thrombotic events might be higher27,35. However, only one dose administered the day before surgery was associated with a decreased rate of blood transfusion in two studies including anaemic patients8,15. In addition, patients currently referred for TAVI have severe aortic stenosis and severe symptoms, and therefore a delay of the intervention might be associated with increased risks. Fourth, and lastly, prior studies frequently used the intravenous route for administering EPO8. However, although subcutaneous administration is associated with a lower peak level than that reached by an intravenous route, slower absorption is associated with higher pre-injection EPO serum concentrations when a subcutaneous route is used17, resulting in fewer doses required without differences in erythropoiesis stimulation, achieving higher cost-effectiveness26.
No increased risk of thrombotic events was observed in this study. Although a higher rate of thrombotic events has been reported with the use of EPO, especially with its chronic use and in patients with higher haemoglobin concentrations35, no increased adverse effects have been reported in the setting of periprocedural surgical use16,17.
Study limitations
The rate of the primary endpoint (RC transfusion rates) was lower than expected. Assumptions for the calculation of the sample size calculation were based on results from previous studies on cardiac surgery and might not be accurate in TAVI populations. However, no clinically significant increase in the haemoglobin level was observed in those patients receiving the EPO, and therefore it is unlikely that any differences between groups would have been obtained by increasing the sample size. Although patients with active bleeding, known anaemia due to aplasia or haemoglobinopathy were excluded, the causes of anaemia and the post-TAVI reticulocyte count were not systematically evaluated. The study might have been underpowered to detect differences in the subgroup analysis. Patients underwent TAVI using older-generation devices; these results might be different when using newer-generation transcatheter heart valves. Despite the randomisation process, there were differences in haemoglobin concentrations at baseline between groups. To overcome this limitation, analyses were adjusted by these differences. The response to EPO might be different in younger and lower-risk patients, hence further studies may be warranted in such patients.
Conclusions
In conclusion, administering EPO (+iron) in anaemic TAVI candidates resulted in modest increments of haemoglobin levels from baseline, yet failed to reduce the rate of RC transfusions or the number of RC units post TAVI. EPO administration in such patients was not associated with increases in thrombotic events or reductions in the rate of acute kidney disease, new-onset myocardial injury, AF or death. Alternative strategies for reducing RC blood transfusion rates in anaemic patients undergoing TAVI warrant investigation.
| Impact on daily practice Red cell transfusions have been associated with an increased risk of early and late mortality following transcatheter aortic valve implantation (TAVI). Preoperative EPO is currently used in these patients to stimulate red blood cell production and reduce the rate of red cell transfusions before cardiac surgery. In the EPICURE trial, administering EPO (+iron) in anaemic TAVI candidates failed to reduce the rate of red cell transfusions or the number of red cell units post TAVI. Alternative strategies warrant investigation. |
Funding
This trial was partially sponsored by unrestricted grants from Edwards Lifesciences, Irvine, CA, USA, and the Foundation of the Quebec Heart and Lung Institute. M. del Trigo, O. Abdul-Jawad Altisent and A. Regueiro were supported by a grant from the Fundacion Alfonso Martin Escudero (Madrid, Spain).
Conflict of interest statement
The authors have no conflicts of interest to declare.

