Abstract
Transcatheter aortic valve implantation (TAVI) has taken the world of cardiovascular therapies by storm. The possibility of implanting aortic valves without recourse to sternotomy or cardiopulmonary bypass has been embraced by cardiologists, surgeons and patients alike as a revolution in management. First performed in 2002 by Alain Cribier1, the technique has exploded into common use during the last three years, such that over 20,000 implants have now been undertaken worldwide. This article discusses complications of TAVI, their avoidance and management.
Introduction
Transcatheter aortic valve implantation (TAVI) has taken the world of cardiovascular therapies by storm. The possibility of implanting aortic valves without recourse to sternotomy or cardiopulmonary bypass has been embraced by cardiologists, surgeons and patients alike as a revolution in management. First performed in 2002 by Alain Cribier1, the technique has exploded into common use during the last three years, such that over 20,000 implants have now been undertaken worldwide. This article discusses complications of TAVI, their avoidance and management.
Pre-procedure
The first and most important aspect in terms of avoidance and management of complications relates to patient selection. Patients who have severe aortic stenosis among a host of comorbidities, such as systemic frailty, renal failure, marked osteoarthritis, chronic obstructive pulmonary disease and cerebrovascular disease, are unlikely to be transformed by aortic valve implantation alone. These patients however formed a substantial proportion of those who underwent the procedure in the early stages. Whether or not they will prove to be the ideal candidates for TAVI in the future remains to be seen.
The multidisciplinary team (MDT) meeting is critical in assessing patient suitability, but only when undertaken in addition to a face-to-face assessment of the patient, discussion of procedural risks and the patient’s expectations and wishes. As yet, no clear frailty index has emerged as the index of choice, and therefore an “eyeball” assessment by at least one member of the MDT is essential if appropriate individuals are to be selected.
The make-up of the MDT is important. Ideally, the group should contain not only implanting cardiologists and surgeons, but also anaesthetists, non-interventional cardiologists, imaging specialists and care of the elderly physicians, who may be best placed to assess the suitability of a given patient for an invasive procedure which still carries a published 30-day mortality of 8-10%2,3. Few MDTs currently consist of the full spectrum of opinion.
The MDT strength currently lies in assessment of anatomical and technical suitability for a given treatment. Thresholds for TAVI therefore vary between units depending on the estimation of likely surgical mortality and morbidity in a given institution. Anatomically, assessment of the aortic annulus and vascular access are key to a successful transcatheter outcome.
During the early phase of a centre’s development, there should be a proctor on site for the case itself.2 Anatomical criteria can be checked with a core lab to ensure that the case is suitable. Both current manufacturers have operated exemplary proctoring schemes, which should serve as a template for introduction of future valvular technology.
Assessment of the aortic annulus is complex, lacking reliability and precision. Transthoracic, transoesophageal echocardiography and CT have all been used and the optimal technique remains unknown.4 The annulus may be elliptical by up to 3 mm5 and therefore aortic dimensions as measured may not prevent significant aortic regurgitation (AR) if the major axis of the annulus is underestimated, or annular rupture if the annulus diameter is overestimated (Figure 1). Bicuspid aortic valves are less suited to TAVI because of commissural fibrosis and calcification, a low-lying non-coronary sinus, and the ensuing risk of valve malposition, annular rupture or significant aortic regurgitation.
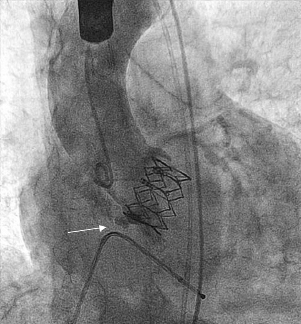
Figure 1. Aortic annular rupture (arrowed).
Assessment of the peripheral vasculature primarily relates to vessel calibre, tortuosity and calcification. For the CoreValve® (Medtronic, Minneapolis, MN, USA), a common femoral artery width of 6 mm is sufficient to allow implantation. Until recently, the Edwards system (Edwards Lifesciences, Irvine, CA, USA) required a common femoral diameter of 8 mm, but this has recently reduced to 6 (18 Fr) to 6.5 mm (19 Fr). Tortuosity or calcification alone may not be prohibitive, but the combination is adverse. Lack of systolic motion of the peripheral vessels may give an indication of rigidity even in the absence of fluoroscopic calcification and is also an adverse feature. The location of the bifurcation of the femoral artery into superficial and profunda arteries and the degree of calcification at the entry site should be used to aid decisions regarding vascular access and closure technique.
Alternative vascular access sites such as the subclavian,6 direct aortic7 and transapical approaches8 should be considered when the iliac vessels are small calibre or tortuous and calcific, when there is peripheral vascular disease or a horizontal aorta (which may make valve alignment problematic).
Sinus of Valsalva width, height of the coronary ostia above the annulus and bulk of native valve leaflets should be assessed to estimate the risk of potentially fatal coronary obstruction.9 Alternative or adjunctive strategies should be planned if necessary. For the Edwards system, a bulky interventricular septum may predispose to valve lift during deployment.
Concomitant coronary artery disease raises the stakes. Need for additional coronary artery bypass grafting increases the overall surgical risk and it may be decided that TAVI alone will suffice. There is no clear consensus on when to undertake coronary artery PCI prior to TAVI,10 however, most units will consider additional PCI when significant disease exists in a proximal location and appears amenable to relatively low risk PCI. Certainly there is consensus that PCI should not be undertaken at the time of TAVI, but rather should be undertaken at least a week prior to TAVI to allow renovascular or other complications to settle prior to TAVI.11
Poorly prepared patients and families may have unrealistic expectations of the risks of TAVI, considering it, wrongly, to be a low-risk intervention akin to coronary artery stenting. A careful and full discussion of the potential risks and benefits is essential to assess whether a given patient understands and gives informed consent.
Specific pre-procedure complications are unusual. However, some pre-procedure actions may limit complications in addition to the precautions discussed above. There is no evidence that clopidogrel preloading is required for transcatheter valve implantation; indeed, the need for clopidogrel at all remains the subject of debate. However, administration of clopidogrel pre-procedure ensures that any vascular complications that occur, such as tamponade or iliac rupture, will be more difficult to manage. It may be sensible therefore to avoid giving clopidogrel as a pre-procedure loading dose. Patients who are at high risk of requiring a pacemaker post-procedure may have their permanent pacemaker (PPM) prior to the valve implantation procedure. For the CoreValve, this includes patients with right bundle branch block (RBBB), or left bundle branch block (LBBB) with first degree or higher grades of pre-existing block. For the Edwards system, no specific recommendations exist as the rate of PPM implantation is much lower (35% vs. 7%)12-14, although significant variation exists between centres.
Procedure
A dedicated cardiothoracic anaesthetist is a key member of the team. Decisions regarding anaesthetic sedation versus general anaesthetic will be made locally. For transfemoral cases, an anaesthetic sedation technique involving low dose remifentanyl/propofol infusion is very successful if there is no competition for the airway space.15 However, if transoesophageal echocardiography (TOE) is being used, or if a subclavian, transaortic or transapical approach is used, general anaesthesia will be preferred. General anaesthesia may expose the patient to additional risk, particularly where poor lung function is a key factor in opting for TAVI in preference to surgery.
Careful choice of vascular access sites for the additional lines can limit risks, such as use of the right internal jugular vein for the temporary wire and the radial artery for the additional pigtail catheter. Entry into the common femoral artery should be undertaken with much greater care than cardiologists are used to. Entering at the mid-point of the femoral head assessed by fluoroscopy ensures that the bifurcation is avoided in 90% of cases.16 Use of the pre-procedure angiogram, or a procedural angiogram from the radial or contralateral femoral approach to guide the precise entry point has additional advantages, with use of an instrument (e.g., needle, Spencer-Wells) overlying the skin to help identify the ideal puncture site. Alternatively, ultrasound-guided access may be used in an attempt to limit vascular complications.
Careful dissection down to the femoral artery will allow optimal deployment of device closure systems such as the Prostar® (Abbott Vascular, Redwood City, CA, USA) or Perclose® (Abbott Vascular). Large bore sheath entry should be made over a stiff wire to avoid inadvertent subcutaneous passage of the sheath. If a large sheath cannot pass, or if there is resistance to valve introduction, pushing the system risks iliac rupture or endarterectomy and should be avoided (Figure 2). The procedure should be terminated and other options considered.
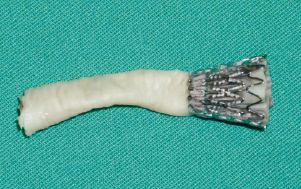
Figure 2. Edwards valve on an endarterectomised section of iliac artery.
Haemorrhage at the access site usually results from injury to the common femoral artery, either because of repeated punctures, tearing of the femoral access site during dissection, or inadvertent entry of the initial sheath or dilating sheaths into the subcutaneous tissue. If the haemorrhage is substantial, control of the bleeding is required and the procedure should be aborted and rescheduled. If vascular rupture occurs, the key to a successful outcome is the immediate recognition of the event. Immediate aortography from the other arterial access site is diagnostic. A soft aortic occlusion balloon should be introduced and inflated to secure the leak and protamine given. Often balloon inflation for 10 minutes at low pressure will be adequate to seal a small leak. Large leaks or tears however require stenting or surgical intervention and immediate liaison with vascular surgery while the aortic occlusion balloon remains inflated is needed. In some cases, placement of a covered stent may stem the flow and resolve the problem (Figure 3).
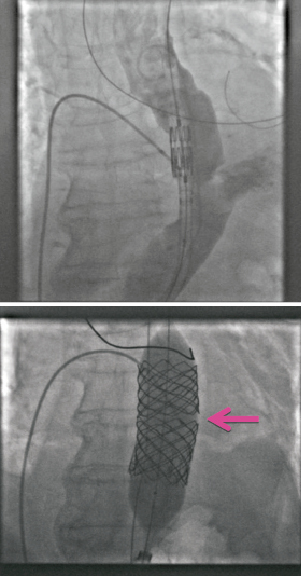
Figure 3. Rupture of thoracic aorta rescued by balloon occlusion and subsequent stent insertion.
Aortography aims to line up the three sinuses of Valsalva. This can be aided by pre-procedural CT, though this increases the overall radiation dose, or by online software applications. For retrograde implantation (e.g., femoral, subclavian) AP caudal 15 degrees is a good place to start, with minor adjustments made according to alignment. For antegrade implantation (e.g., transapical) caudal views foreshorten the route from apex to valve and therefore LAO cranial projections are usually preferred. In finding the optimal view, small volume contrast injections should be used to minimise the risk of contrast nephropathy.11
Crossing the aortic valve should be a gentle technique using a soft-ended straight wire, usually through an Amplatz Left 1 catheter –if the direction of the wire is correct it will pass straight through, otherwise it will deviate and pushing it further will only risk injury, stroke or coronary artery damage. Once the valve is crossed, transcatheter gradient and left ventricular end-diastolic pressure (LVEDP) should be measured. Placement and curvature of a stiff 0.035 wire in the ventricle is very important to avoid tamponade. The wire should be J-tipped and should be hand-shaped into a wide-curved pigtail to allow it to pass unrestricted and coil up in the ventricle while also offering maximum support. From the transapical approach, crossing the aortic valve is straightforward with a J-tipped 0.035 wire from the apex, and can then be exchanged over a JR4 catheter for an Amplatz Super Stiff™ (Boston Scientific, Natick, MA, USA) wire positioned in the descending aorta.
Balloon valvuloplasty should be undertaken using a balloon 0-2 mm smaller than the measured aortic annulus. Dilatation should occur during rapid temporary pacing, sufficient to reduce the pressure to 40mmHg systolic. The temporary wire itself should be a soft-tipped catheter of small gauge (e.g., 4 Fr). Larger-bore temporary wires can pass through the right ventricle and have caused fatal cardiac tamponade in a small number of cases. The balloon used for valvuloplasty should be sufficiently robust to have minimal risk of bursting and offer maximum expansion at the level of the aortic valve. Balloon bursting, with resulting ventricular, coronary or peripheral debris have been reported (Figure 4) and a balloon with a rated burst pressure of ≥4 atm is recommended. Repeat balloon valvuloplasty inflations may help to crack aortic valve calcification and optimise expansion of the valve; though at present there is no consensus on whether a minimalist or therapeutic approach to the valvuloplasty is best.
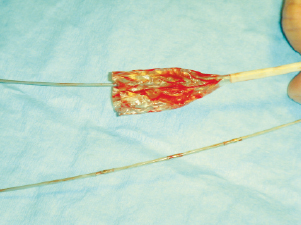
Figure 4. Circumferential dehiscence of aortic valvuloplasty balloon.
Deployment of the valve varies according to the valve type. The CoreValve should be aligned with the lower aspect of the non-coronary sinus and should be deployed slowly to allow the valve to fix itself 2-6 mm below the aortic annulus. This is particularly true for implantation into existing bioprosthetic valves. The rigid ring of the bioprosthesis will prevent repositioning of the valve once the skirt has opened.17 Placement at this level minimises the risk of aortic regurgitation and is thought to reduce the need for a permanent pacemaker.13 The Edwards valve should be carefully aligned with the mid-portion of the aortic annulus and should be deployed under rapid pacing sufficient to reduce the pressure to <40 mmHg. Deployment is also guided by online TOE, and balloon inflation can be gradual rather than rapid to allow slight alteration in final position of the valve. The sequence of instructions from the team leader should be precise in order to ensure that valve deployment occurs appropriately. For the CoreValve system, implantation during hypertension (>150 mmHg) may increase the risk of valve expulsion. Pacing at a rate to bring the systolic pressure down to 100 mmHg may be used to limit this risk.
Once the valve is deployed, the guide catheter and wire should be removed and an assessment of the result made. Aortic regurgitation is difficult to assess by aortography, as it varies with the height of the pigtail and the volume of contrast used. Equally, immediate echocardiography can sometimes be unreliable, as any leak may be circumferential and narrow width, and may be therefore underestimated echocardiographically. These components however remain the gold standard assessments of AR post TAVI. Key additional components of the assessment of immediate severe AR are the LVEDP, the aortic diastolic pressure and the presence or absence of a clear dicrotic notch in the aortic pressure waveform, indicative of competent aortic valve closure.
Severe aortic regurgitation is a marker of adverse outcome18. The mechanism and location (central or paravalvular) need to be understood. For the CoreValve this is likely to relate either to non-circumferential expansion of the valve, a low placement of the valve, or, rarely, a high placement of the valve. Treatment varies according to the mechanism. For a low-placed valve, eyelet snaring and lifting can resolve the problem but is also unpredictable and can result in valve migration. Placement of a second valve in a higher position is straightforward but expensive. Nitinol frame expansion during the first 10 minutes may reduce AR significantly so if the CoreValve appears in a good position this should be awaited first. If however AR persists or the valve appears elliptical on echocardiography, post-dilatation of the valve can be done with good effect. For the Edwards valve, AR may rarely occur due to a non-functioning leaflet. This can sometimes be corrected by mechanical manipulation of the leaflet with a pigtail catheter. Alternatively, AR may be paravalvular or due to misplacement of the valve or incorrect sizing. These can be corrected by placement of a second valve or repeat dilatation of the valve.
Coronary occlusion can occur if the valve and associated native leaflets are too bulky for the width of the sinuses of Valsalva. If there is concern about this possibility, a pre-implant balloon valvuloplasty with simultaneous associated aortography may demonstrate whether coronary occlusion is likely to occur. If aortography during balloon inflation does not demonstrate coronary perfusion (Figure 5) the procedure should be terminated and other options considered. If there is persisting hypotension after valve implantation, either tamponade or coronary occlusion should be immediately suspected. Echocardiography and aortography are key to this process. If the left coronary ostium is occluded (Figure 6), the valve should immediately be snared and pulled up out into the ascending aorta to allow coronary perfusion to be re-established. Once this is achieved, a decision can be taken about placement of a second valve, or abortion of the procedure.
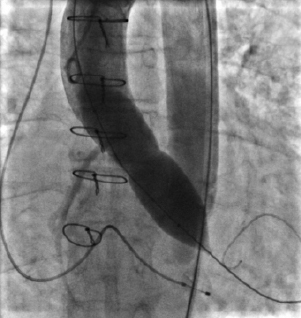
Figure 5. Occluded left main stem during balloon dilatation of the aortic root.
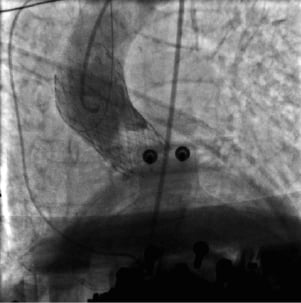
Figure 6. Occlusion of left main stem after valve implantation (Lucas device in place).
Two grades of valve embolisation exist. In the first grade (applicable to CoreValve only) the valve remains attached to the delivery system but has risen above the aortic valve. In this case it can be withdrawn around the aorta, into the iliac system and sheath, and re-sheathed within the delivery system to allow repeat placement.19 Pulling the open valve around the aorta is likely to be a risk factor for stroke. In the second grade, the entire valve is inadvertently deployed in a supra-aortic position. In these cases, it needs to be lifted sufficiently far above the aortic valve to allow a second valve to be placed. This can usually be achieved with one or more snares (Figure 7) and the valve left in situ in the ascending aorta (CoreValve) or descending aorta (Edwards). In the case of the Edwards valve, the wire should remain in situ to ensure that the valve cannot invert and become obstructive.
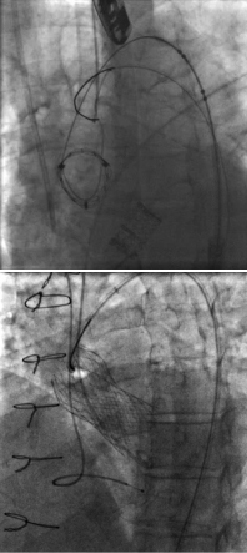
Figure 7. Valves snared and lifted into ascending aorta.
Hypotension post-implantation persists in a proportion of cases and the mechanism must be quickly understood as hypotension may rapidly lead to a spiral of decay. Immediate transthoracic or transoesophageal echocardiography should be made to assess for tamponade, ventricular function, interference with the mitral valve and degree of aortic regurgitation, along with aortography to assess coronary perfusion. Treatment must be rapidly initiated to ensure that coronary perfusion pressure is re-established. Chest compressions and/or vasopressors/inotropes such as milrinone or adrenaline should be used early while the cause is sought and treated. Tamponade is a feared and potentially fatal event during TAVI. The timing of the development of tamponade should give the clue as to its aetiology. Causes can include perforation of the right ventricular apex by a temporary pacing wire, perforation of the left ventricle by the super stiff guidewire, or annular rupture due to incorrect sizing or over-sized balloon dilatation. In all cases, immediate pericardiocentesis will initially stabilise the haemodynamics and allow time to plan further treatment. Patients with annular rupture however rarely survive.
If hypotension occurs unexpectedly, establishment of femoral cardiopulmonary bypass can be an effective way to permit stabilisation of the patient and many authorities believe that all units undertaking TAVI should have cardiopulmonary bypass capability immediately on hand and primed for use. Opening the chest in the lab is fortunately a rare event, but can be life-saving. Usually a decision should have been made prior to TAVI as to whether the chest will be opened in the event of a dire emergency.
The procedure is not over until the fat sheath is removed and haemostasis secured. As with surgical procedures, vascular closure during hypertension is not recommended. Pacing at a rate sufficient to lower the blood pressure to 80-100 mmHg may be optimal for five minutes to allow successful haemostasis. For most CoreValve implants, closure is successfully done with the Prostar® or dual Perclose® percutaneous suture device. This is also now true for the Edwards system, which used to be larger caliber and require surgical closure. For both systems, a final angiogram from the radial or contralateral femoral artery is helpful to ensure complete haemostasis has been achieved. For transapical delivery, closure is made under direct visualisation and relative hypotension may also be helpful.
Post-procedure
General anaesthesia or anaesthetic sedation should be withdrawn as soon as possible, often in the catheter lab itself, in order to allow early recovery and mobilisation. Once the patient is back on the coronary care unit or high dependency unit, they need close monitoring and assessment for the first few hours. Hypotension should be vigorously treated early to avoid complications resulting from cerebral, coronary or renal hypoperfusion. Causes should be sought, especially tamponade or bleeding, and, if necessary, fluids or blood given to restore haemodynamics. Continued invasive monitoring of intra-arterial pressure for the first two hours post-procedure is recommended to ensure that hypotension is diagnosed early, and to assess blood gases for hypoventilation, potassium levels and acid/base balance.
Stroke is a feared complication of TAVI, which fortunately occurs relatively rarely (2-3%)12, but is usually only diagnosed post-procedure. Meticulous attention to de-airing and activated clotting times during the procedure will limit the risk, but brain lesions on MRI are frequently seen post-procedure20 and future work will need to be directed to reduction of stroke rates. Standardisation of the definition of stroke according to the Valve Academic Research Consortium will help reduce variability in reporting.21
The temporary pacing wire should be removed early if possible. For Edwards patients, the wire can usually be removed at the end of the procedure unless there is high grade atrioventricular block. For CoreValve patients, if there is no LBBB, or if there is LBBB but a normal PR interval, the wire can be removed on the evening of the procedure to reduce risks of infection and facilitate immediate mobilisation. The patient however needs to be monitored continuously for five days to assess for gradual development of heart block. If the patient has LBBB with a PR interval of >280 ms, or has had any higher degree of block, a PPM should be scheduled for the next day.14
Occasional patients develop acute pulmonary oedema shortly after the procedure despite an optimal result. This often seems unfair and difficult to explain. However, assessment of the LVEDP post-procedure may warn of its impending development due to lack of diastolic compliance of the left ventricle, and in this case, diuretics and vasodilators can be effective in prevention. Post-procedure hypertension is also common, due to removal of outflow obstruction, and can be managed in the first 24 hours with a titrated nitrate infusion.
A ward round at the end of the implantation day by a team comprising a minimum of the cardiologist and anaesthetist is strongly recommended to ensure that problems are foreseen and treated appropriately. Management of such “post-operative” patients is an area with which the cardiothoracic anaesthetist is likely to be more familiar than the cardiologist.
Conclusions
TAVI has emerged as a highly effective treatment for aortic valve disease in patients at high operative risk. Valve longevity remains unknown and therefore extrapolation into lower risk cohorts should be cautious. Avoidance and management of complications begins most importantly with a face-to-face meeting with the patient, and a full discussion at an MDT staffed by representatives from different disciplines. Preparation for possible problems remains all-important. With good initial proctoring, and subsequent standalone experience, teams will develop the skills to recognise and manage unforeseen complications quickly and effectively in the majority of cases.
Acknowledgments
Figures 1,2 and 3 are reproduced with thanks from submissions to London Valve Live meeting 2010.
Conflict of interest statement
Drs Redwood, Young, MacCarthy and Thomas are proctors for Edwards; Drs Hildick-Smith, Brecker, Blackman and Manoharan are proctors for Medtronic. The other authors have no conflicts of interest to declare.

