Introduction
In the current guidelines for acute coronary syndrome (ACS), the GRACE risk score is recommended for risk stratification. The GRACE risk score 2.0 provides the risk of death and myocardial infarction (MI) after ACS, but it does not provide a bleeding risk prediction. The PRECISE-DAPT and PARIS scores were developed from patient populations treated with percutaneous coronary intervention (PCI) regardless of their clinical presentations. They predict out-of-hospital bleeding risk. The PARIS score also provides a thrombotic risk prediction. As a tool for mortality prediction, the logistic clinical SYNTAX score was redeveloped from the GLOBAL LEADERS trial12. Recently, the PRAISE score, based on a machine-learning algorithm, was developed for the combined prediction of death, MI, and major bleeding one year after discharge in ACS patients3. These risk prediction models have been introduced into clinical practice, but their performances have not been compared (Supplementary Table 1).
Methods
We applied five scores to ACS patients treated with PCI in the GLOBAL LEADERS trial for the assessment of all-cause death, MI defined by the third universal definition, and Bleeding Academic Research Consortium (BARC) type 3 or 5 major bleeding at one year after discharge2. The GLOBAL LEADERS trial investigated aspirin-free antiplatelet treatment after PCI (experimental arm: 1-month dual antiplatelet therapy [DAPT] followed by 11-month ticagrelor monotherapy vs control arm: 12-month DAPT) in an all-comers population. Out of 15,968 patients, 7,457 presented with ACS and were treated with PCI. Thirty-one patients died before discharge. The database of 7,426 patients who were discharged has been used for the validation of death prediction. Nineteen patients were lost to follow-up for MI and bleeding and only vital status in these patients was available after discharge. Thus, the data of 7,407 patients were used for the validation of MI and bleeding prediction. Details of score calculation are provided in Supplementary Appendix 1. The discriminative abilities were assessed using Harrell’s C statistic, and integrated discrimination improvement (IDI). The calibration was evaluated using the Hosmer-Lemeshow goodness-of-fit statistical test. Regarding the PRAISE score, agreement between observed and predicted rates was assessed by calibration plots, and Brier scores were reported as overall measures of performance.
Results
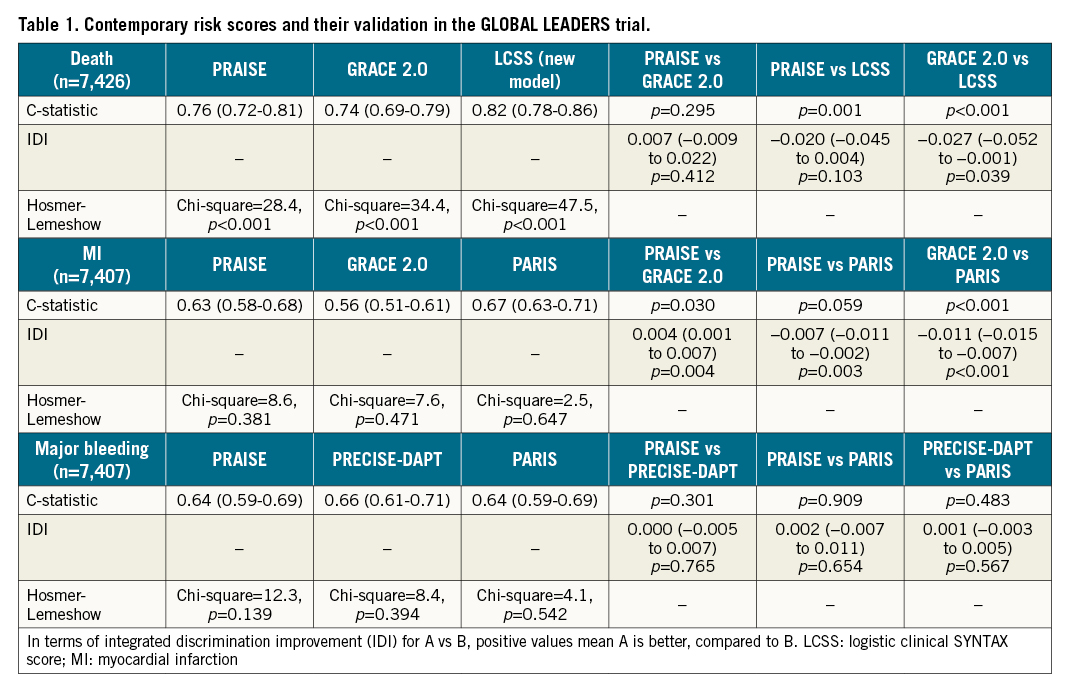
One year after discharge, all-cause death, MI, and BARC type 3 or 5 major bleeding occurred in 103 patients (1.4%), 140 patients (1.9%), and 111 patients (1.5%), respectively. The numbers of missing variables for score calculation are presented in Supplementary Table 2. C-statistics, IDIs and the results of the Hosmer-Lemeshow test are summarised in Table 1, Supplementary Figure 1 (overall population), Supplementary Table 3 and Supplementary Figure 2 (the experimental and control arms). In all models predicting death, calibrations based on the Hosmer-Lemeshow tests were poor. The calibration plots and Brier scores for the PRAISE score are shown in Supplementary Figure 3 and Supplementary Figure 4.
Discussion
All-cause mortality may be viewed as the ultimate endpoint; however, MI and major bleeding have significant bearing on subsequent mortality4. Two separate risk scores for bleeding events and thrombosis events provide not only risk stratification but also trade-off between ischaemic and bleeding risks, which supports the individual optimal antiplatelet and anticoagulant therapy5.
The predictivities in the development cohorts can be higher than those observed in the external validation cohort, which may depend on the eras of development and external cohorts. Advances in medicine could have an influence on the predictivity. Of note, observed versus predicted rates of the PRAISE score in the control arm were well calibrated according to calibration plots but, in the experimental arm, the PRAISE score overestimated the rates of death and bleeding (Supplementary Figure 4). Therefore, external validation using contemporary data should be performed.
The results stemming from the GLOBAL LEADERS trial demonstrated that the PRAISE score could provide combined predicted event rates of death, MI, and bleeding with useful or helpful discrimination. Calibration based on Hosmer-Lemeshow tests was poor for the prediction of death. Thus, improved risk scores are still warranted. The predictive values of the PRAISE score were at least non-inferior to those of conventional statistical models when the models were externally validated (Table 1), which suggests that using machine learning approaches might have some promise in risk prediction.
Limitation
Outcomes predicted by the scores are different in the timing (i.e., at 1 year vs 2 years, etc.) and criteria for events (i.e., BARC vs Thrombolysis In Myocardial Infarction [TIMI] bleeding criteria, etc.); therefore, calibration plots and Brier scores were evaluated only for the PRAISE score. The logistic clinical SYNTAX score was developed from the GLOBAL LEADERS trial to predict death occurring post procedure up to two years (internal validation). MI and bleeding were site-reported and were not centrally adjudicated due to limited financial resources.
Conclusion
The PRAISE score provides the predicted rates of death, MI, and major bleeding one year after discharge in ACS patients with useful or helpful discrimination, which will support the individual optimal antiplatelet and anticoagulant therapy.
Funding
The GLOBAL LEADERS study was sponsored by the European Clinical Research Institute, which received funding from Biosensors International, AstraZeneca, and The Medicines Company.
Conflict of interest statement
H. Hara reports a grant for studying overseas from the Japanese Circulation Society, a grant-in-aid for JSPS Fellows and a grant from the Fukuda Foundation for Medical Technology. P.W. Serruys reports personal fees from Sino Medical Sciences Technology, Philips/Volcano, Xeltis, HeartFlow, Meril Life, and SMT outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

