Abstract
Background: Bleeding is the principal safety concern of antithrombotic therapy and occurs frequently among patients with coronary artery disease (CAD).
Aims: We aimed to evaluate the prognostic impact of bleeding on mortality compared with that of myocardial infarction (MI) in patients with CAD.
Methods: We searched Medline and Embase for studies that included patients with CAD and that reported both the association between the occurrence of bleeding and mortality, and between the occurrence of MI and mortality within the same population. Adjusted hazard ratios (HRs) for mortality associated with bleeding and MI were extracted and ratios of hazard ratios (rHRs) were pooled by using inverse variance weighted random effects meta-analyses. Early events included periprocedural or within 30-day events after revascularisation or acute coronary syndrome (ACS). Late events included spontaneous or beyond 30-day events after revascularisation or ACS.
Results: A total of 141,059 patients were included across 16 studies; 128,660 (91%) underwent percutaneous coronary intervention. Major bleeding increased the risk of mortality to the same extent as MI (rHRsbleedingvsMI 1.10, 95% CI: 0.71-1.71, p=0.668). Early bleeding was associated with a higher risk of mortality than early MI (rHRsbleedingvsMI 1.46, 95% CI: 1.13-1.89, p=0.004), although this finding was not present when only randomised trials were included. Late bleeding was prognostically comparable to late MI (rHRsbleedingvsMI 1.14, 95% CI: 0.87-1.49, p=0.358).
Conclusions: Compared with MI, major and late bleeding is associated with a similar increase in mortality, whereas early bleeding might have a stronger association with mortality.
Introduction
Bleeding is the principal safety concern in patients with coronary artery disease (CAD) receiving long-term antithrombotic therapy and the most common non-cardiac adverse event among those undergoing percutaneous coronary intervention (PCI). Single or dual antiplatelet therapy in patients with chronic atherosclerotic vascular disease carries a risk of major bleeding of 1-2% annually1,2. Moreover, in the setting of PCI, major bleeding occurs in 1-2% of patients periprocedurally and up to 4% at one year in the absence of high bleeding risk features3,4.
There is a growing body of evidence showing that bleeding has a negative prognostic impact with a heightened risk of mortality5. Several randomised trials, performed in the chronic setting, showed a decreased risk of ischaemic complications by means of more potent antiplatelet therapies without a concomitant reduction in the risk of mortality. Arguably, the excess of clinically relevant bleeding with more effective antithrombotic regimens could erode their ischaemic benefit, resulting in a shift of the risk for all-cause mortality towards the null effect. However, although the trade-off between ischaemia and bleeding is increasingly recognised6, it remains unclear whether the excess of mortality risk conferred by bleeding is of comparable magnitude to that ensuing after myocardial infarction (MI). Furthermore, whether differences between late and early events exist has not yet been determined. Therefore, we undertook a systematic review and meta-analysis to summarise the available evidence on the relative prognostic association of bleeding and MI with mortality in patients with CAD treated medically or with PCI.
Methods
SEARCH STRATEGY AND SELECTION CRITERIA
Studies had to include participants with CAD irrespective of their management (either medically or with PCI) and had to determine both the association between the occurrence of bleeding and mortality, and that between the occurrence of MI and mortality within the same population. Studies providing only the association between bleeding and mortality or, alternatively, only the association between MI and mortality were excluded. All studies had to adjust at least for age.
Studies were identified by a systematic search in Medline and Embase from each database inception to January 2021. The detailed search algorithm is provided in Supplementary Appendix 1. Reasons for exclusion were discussed and discrepancies were resolved by consensus.
The study protocol was registered in PROSPERO (CRD42020154931).
DATA ANALYSIS
Data were independently extracted by two different reviewers (A. Oliva, M. Avvedimento). We extracted adjusted hazard ratios (HRs) with 95% confidence intervals (95% CI) for the risk of mortality associated with different types of bleeding and MI, extracting HRs with 95% CI by bleeding severity (i.e., major vs minor) and setting of bleeding and MI (i.e., early vs late). Early events were defined as periprocedural events or, alternatively, as events occurring within 30 days after revascularisation or acute coronary syndrome (ACS). Late events were defined as spontaneous events or, alternatively, as events occurring beyond 30 days after revascularisation or ACS. In view of the anticipated variation among studies in the bleeding definitions used, the Bleeding Academic Research Consortium (BARC) classification took precedence over Thrombolysis In Myocardial Infarction (TIMI), followed by Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries (GUSTO), and the primary classification specified in the study’s protocol in case neither BARC, TIMI nor GUSTO classifications were used. BARC type 3 bleeding was used as a reference to define major bleeding, and other definitions were mapped accordingly. For example, TIMI minor and major bleedings were both considered major bleedings, whereas TIMI minimal bleedings were considered minor (Supplementary Table 1-Supplementary Table 3). In addition to the covariate of age, information was extracted on additional variables, if any, considered for deriving the adjusted HRs (Supplementary Table 4). Two reviewers independently assessed the risk of bias of studies using a modified version of the Newcastle-Ottawa scale score7, based on three selection criteria and three outcome criteria. We considered studies that met four or more of these Newcastle-Ottawa scale criteria to be of higher quality.
STATISTICAL ANALYSIS
The ratio of hazard ratios (rHRs) was used as a measure of the strength of the association between bleeding and MI in terms of mortality risk (Supplementary Appendix 2). An rHRsbleedingvsMI >1 indicates a stronger association of bleeding with mortality as compared with MI (i.e., higher risk of death with bleeding), whereas an rHRsbleedingvsMI <1 indicates a stronger association of MI with mortality, as compared with bleeding (i.e., higher risk of death with MI)8. HRs with 95% CI as well as their ratios were pooled with inverse variance weighted random effects meta-analysis. HRs for the associations of bleeding and MI with mortality were derived from the same participants and correlated. We therefore calculated the median design factor, defined as standard error derived after accounting for correlation within participants divided by crude standard error, in the only study that appropriately derived ratios of HRs and allowed the calculation of the corresponding crude standard error9. This median design factor of 1.041 was used to inflate the crude standard errors of rHRs of the remaining studies. We accounted for different types of bleedings and MIs, separately analysing the HRs for early and late events, and for the composite of early or late events. We also accounted for bleeding severity, analysing HRs for major bleeding or, if not available, for the composite of major or minor bleeding separately from HRs for minor bleeding events only. Sensitivity analyses were then performed by excluding outlying studies (outside the boundaries of Galbraith plots), between studies published before versus after 2015, as well as between randomised and non-randomised studies. We determined heterogeneity between trials using the I2 statistic, with estimates near 25% indicating a small, near 50% a moderate, and near 75% a large extent of heterogeneity. Studies contributing to significant heterogeneity were identified by Galbraith plots in which the estimated effect of each study was plotted against its precision10. Sensitivity analyses were then performed by excluding outlying studies (outside the boundaries of Galbraith plots). All p-values are two-sided. Analyses were performed in Stata Release 14 (StataCorp, College Station, TX, USA).
Results
We screened 5,571 unique citations and deemed 17 reports related to 16 studies eligible after full-text review, including 141,059 participants9,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25. Table 1 shows the main features of the studies selected for analysis. The definitions of bleeding and MI used in each study are reported in Supplementary Table 1 and Supplementary Table 5.
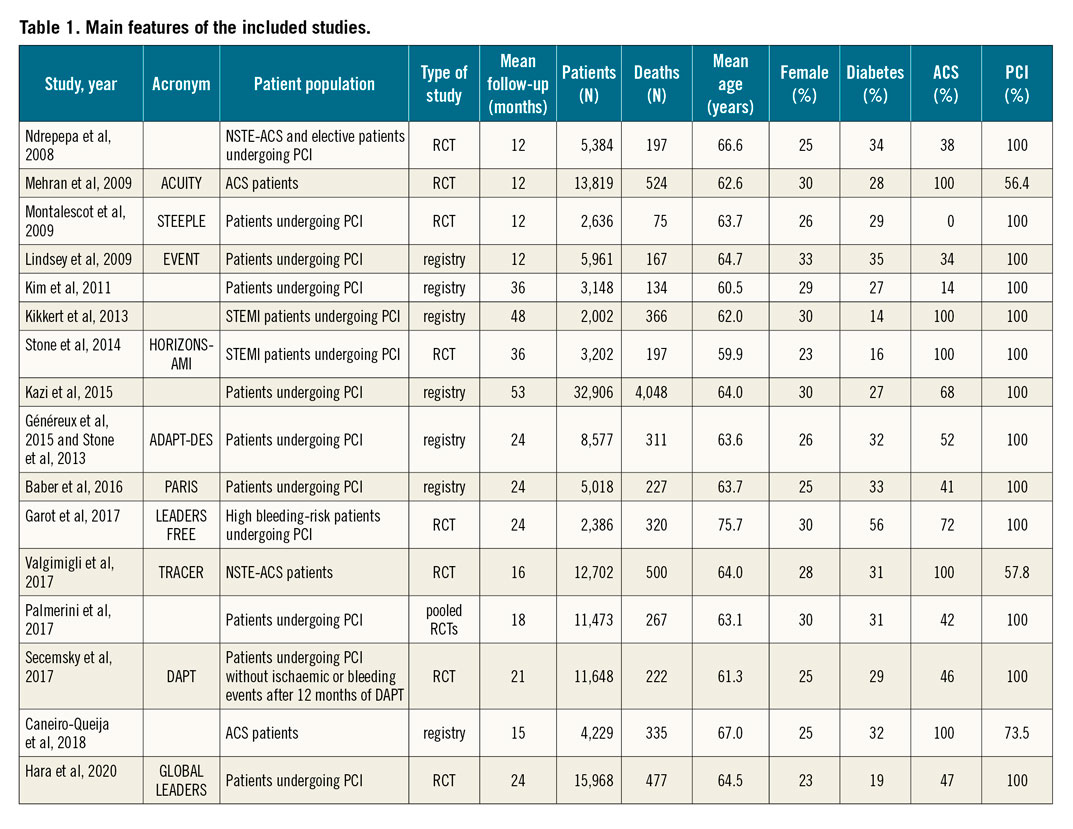
Studies were published between 2008 and 2020 and had a median follow-up of 22.5 months (interquartile range [IQR] 13.5-30). Median age was 63.7 (IQR 62.3-64.7) years and 27.7% of participants were female. The proportion of participants undergoing PCI or with ACS was 91% (N=128,660) and 63% (N=88,867), respectively. Procedural characteriscs of studies are reported in Supplementary Table 6. A total of 8,367 deaths occurred in 141,059 participants, with a median risk of 3.9% across 16 studies (IQR 2.9%-7%). Bleeding and MI event rates reported across the studies are displayed in Supplementary Table 7.
MAJOR BLEEDING (±MINOR BLEEDING) vs MI
RISK OF ALL-CAUSE MORTALITY AFTER EARLY OR LATE EVENTS
HRs for the composite of early or late events after PCI were available for eight studies (n=51,780 participants). The median risk of bleeding was 6.2% (IQR 1.9%-8.5%; 2,024 events/49,778 participants; 7 studies) and the median risk of death among participants with bleeding was 17.8% (IQR 14.6%-19%; 258 deaths/1,630 participants; 5 studies). The median risk of MI was 6.5% (IQR 3.1%-7.4%; 1,804 events/40,307 participants; 7 studies) and the median risk of death among participants with MI was 13.4% (IQR 10.4%-29.2%; 267 deaths/1,600 participants; 6 studies). The risk of all-cause mortality was significantly increased among participants with early or late bleeding (HR 4.44, 95% CI: 3.02-6.52) and MI (HR 4.10, 95% CI: 3.34-5.03). The rHRs for bleeding versus MI were not significant (rHRsbleedingvsMI 1.10, 95% CI: 0.71-1.71, p=0.668) (Central illustration, Figure 1, Figure 2). Heterogeneity was high for relative risks (I2=89% and I2=58%, for bleeding and MI, respectively) and their ratio (I2=81%). However, after the exclusion of outlying studies, the ratio of HRs remained not significant (rHRsbleedingvsMI 1.03, 95% CI: 0.80-1.32, I2=19%) (Table 2).
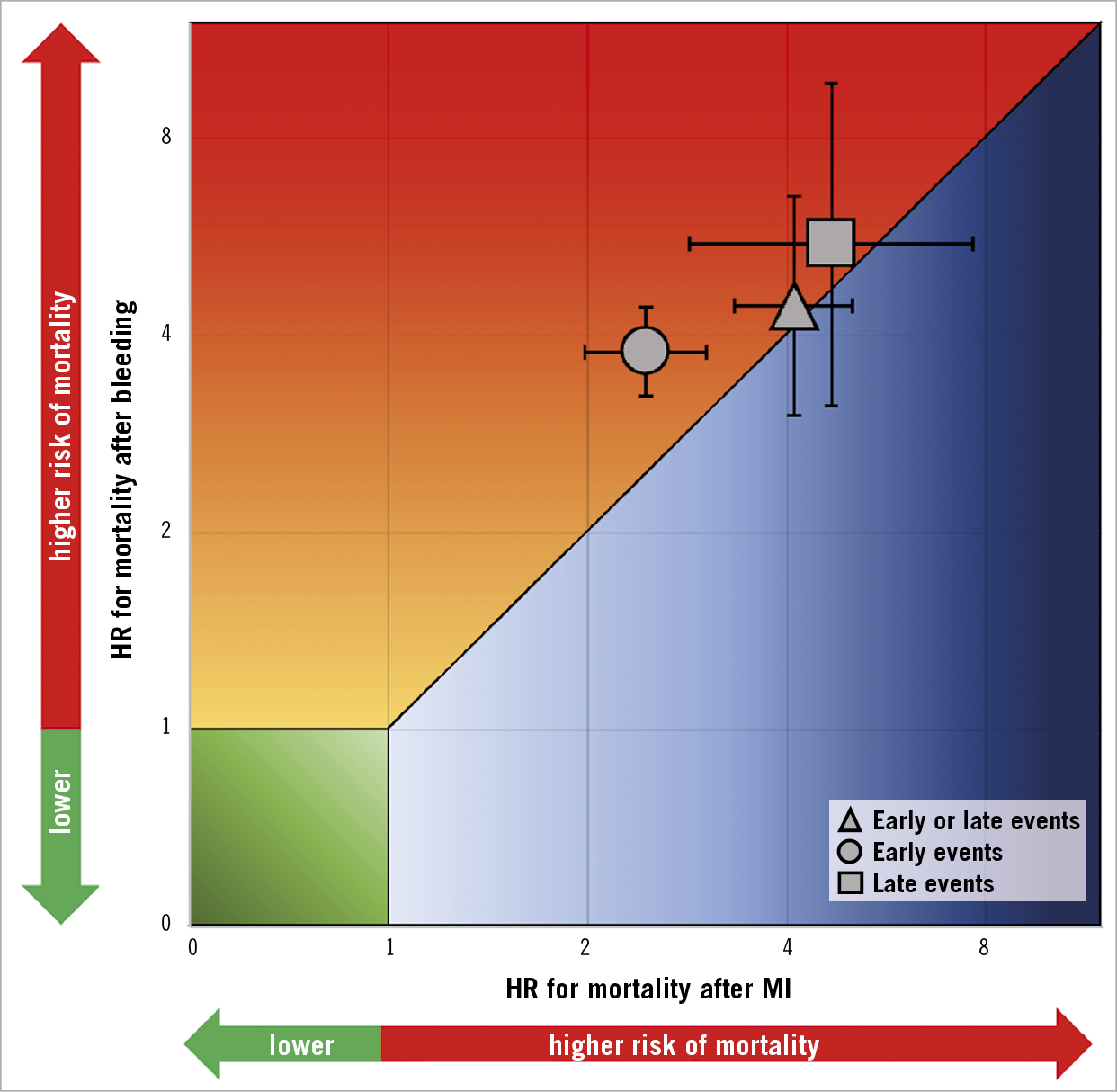
Central illustration. Association between bleeding and myocardial infarction with mortality for early events, late events and early or late events.
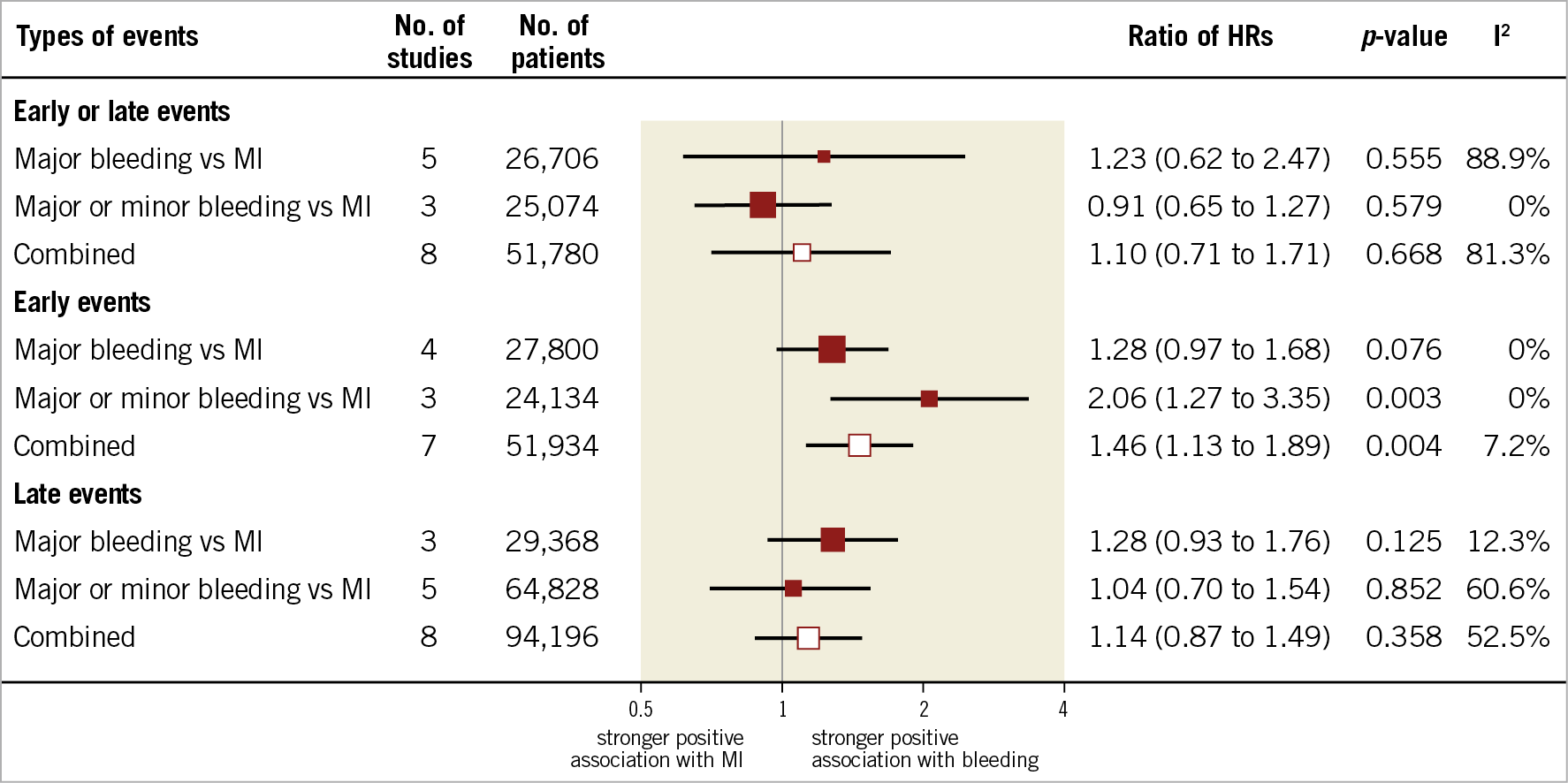
Figure 1. Association of bleeding and myocardial infarction with mortality in patients with coronary artery disease treated with medical therapy or percutaneous coronary intervention.
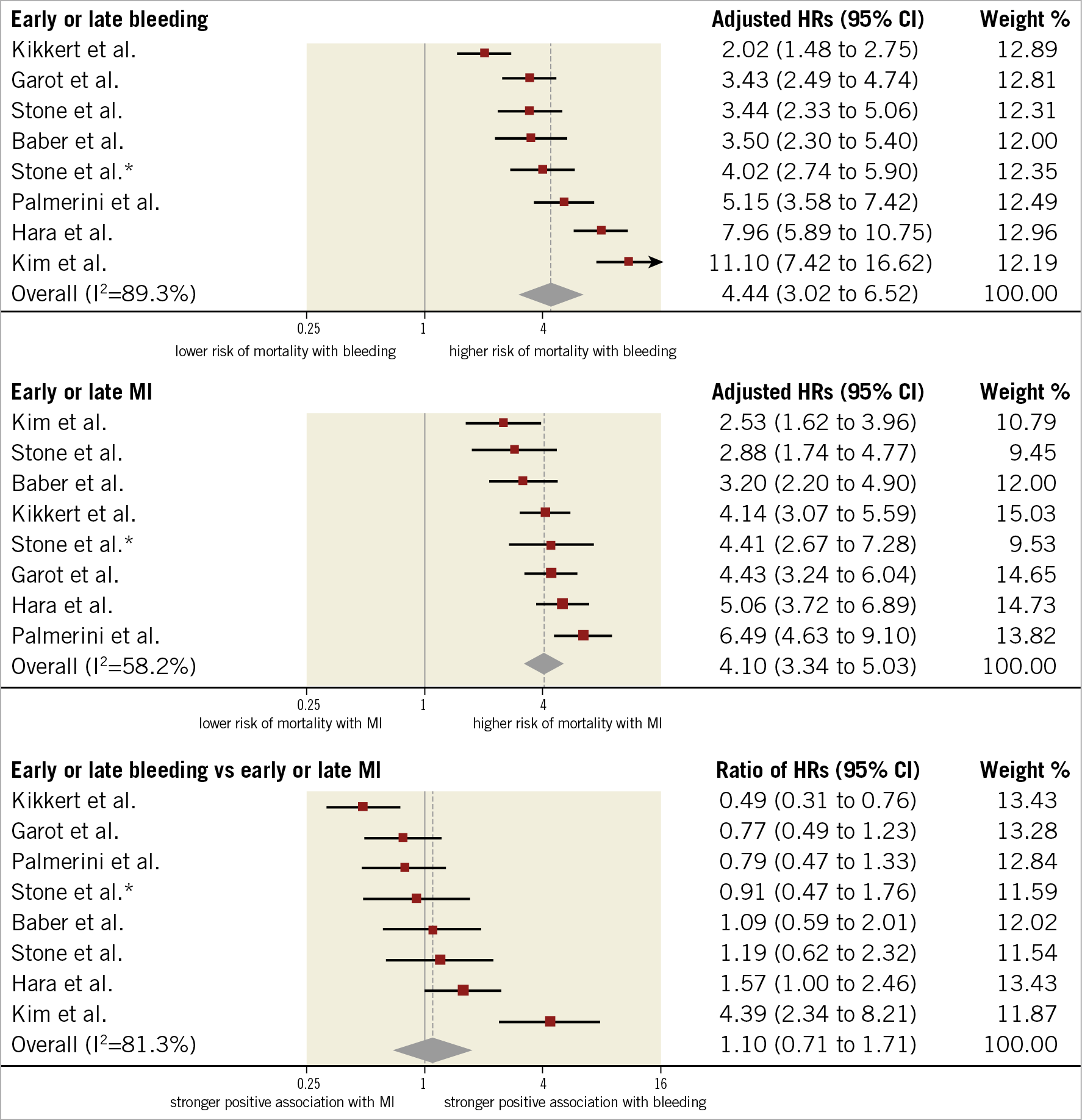
Figure 2. Adjusted hazard ratios for all-cause mortality for patients with early or late events. HRs for bleeding are for major bleeding. *Refers to Stone et al 2013.
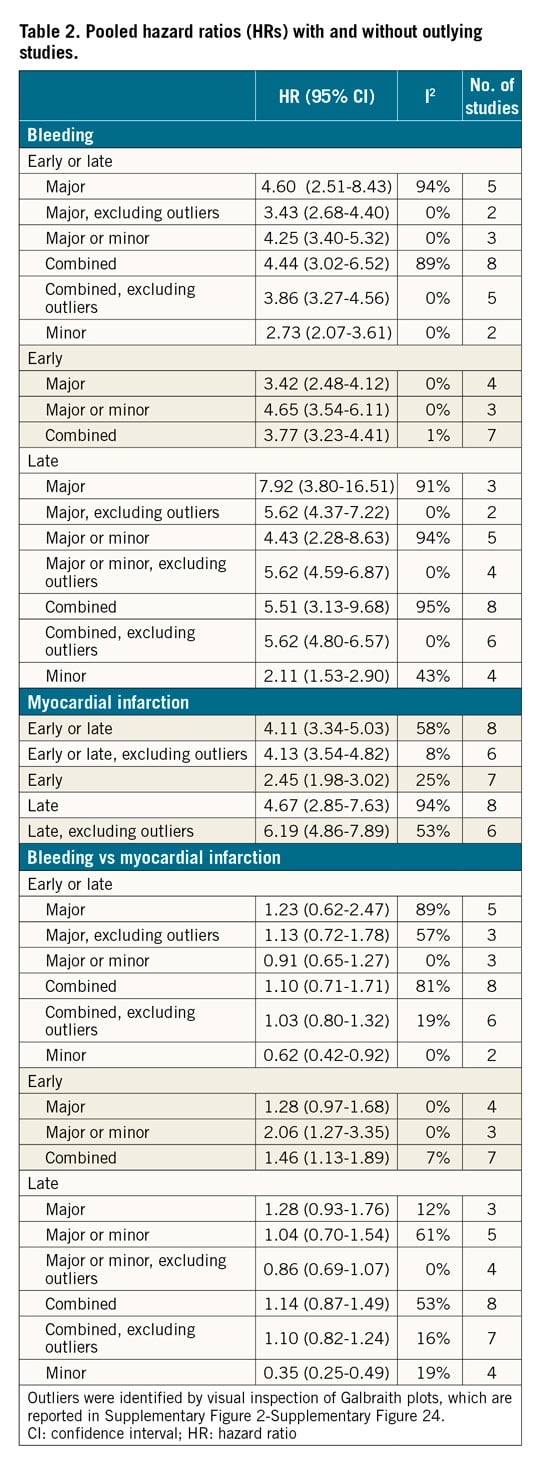
RISK OF ALL-CAUSE MORTALITY AFTER EARLY EVENTS
HRs for early events were available for seven studies in 51,934 participants undergoing cardiac catheterisation and/or PCI. The median risk of early bleeding was 3.5% (IQR 1.5%-4.3%; 1,439 events/49,928 participants; 6 studies with available data) and the median risk of death was 15.3% (IQR 14.4%-15.6%; 216 deaths/49,928 participants; 6 studies). The median risk of early MI was 5.2% (IQR 1.7%-5.8%; 1,836 events/49,928 participants; 6 studies) and the median risk of death was 8.6% among participants with early MI (IQR 8.1%-10.9%; 153 deaths/1,671 participants; 5 studies). The risk of all-cause mortality was increased to a greater extent after early bleeding (HR 3.77, 95% CI: 3.23- 4.41; I2=1%) than early MI (HR 2.45, 95% CI: 1.98-3.02; I2=25%), resulting in a significant rHRs (rHRsbleedingvsMI 1.46, 95% CI: 1.13-1.89, p=0.004; I2=7%) (Central illustration, Figure 2, Figure 3).
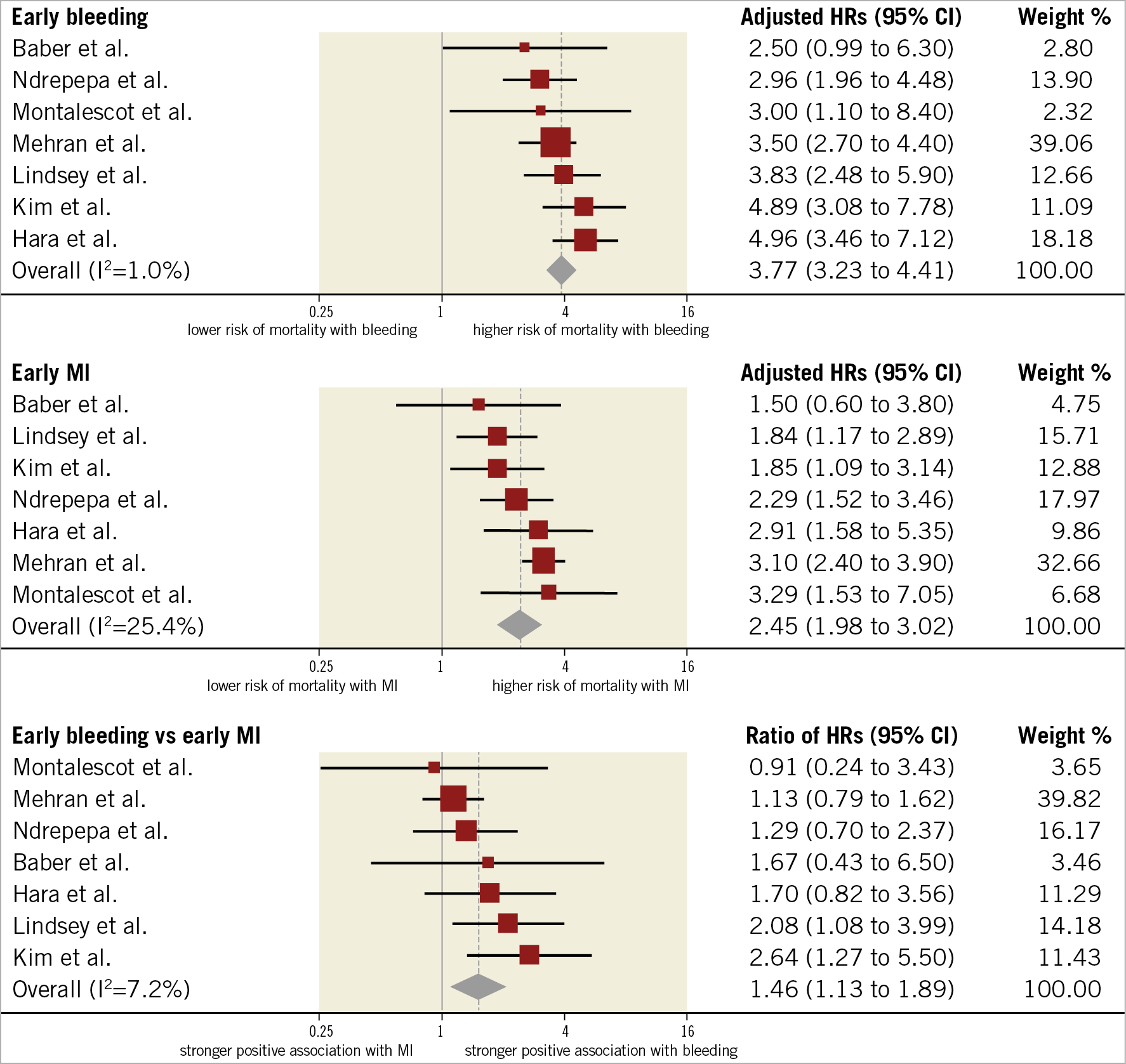
Figure 3. Adjusted hazard ratios for all-cause mortality for patients with early events. HRs for bleeding are for major bleeding.
RISK OF ALL-CAUSE MORTALITY AFTER LATE EVENTS
HRs for late events were available for eight studies in 94,196 participants. The median risk of late bleeding was 3.1% (IQR 2.2%-5.8%; 3,261 events/94,196 participants; 8 studies) and the median risk of death among participants with late bleeding was 17.6% (IQR 13.4%-22.1%; 557 deaths/3,261 participants; 8 studies). The median risk of late MI was 2.9% (IQR 2.5%-4.7%; 3,150 events/94,196 participants; 8 studies) and the median risk of death among participants with late MI was 17.2% (IQR 10.9%-21.3%; 571 deaths/2,724 participants; 6 studies). As shown in Figure 4, the relative risk of all-cause mortality was significantly increased after late bleeding (HR 5.51, 95% CI: 3.13-9.68; I2=95%) and late MI (HR 4.67, 95% CI: 2.85-7.63; I2=94%). The rHRs was not significant (rHRsbleedingvsMI 1.14, 95% CI: 0.87-1.49, p=0.358; I2=53%) (Central illustration, Figure 2, Figure 4), suggesting a similar impact of the two events on mortality. The rHRs remained similar after the exclusion of the studies that were identified as outliers at visual inspection of Galbraith plots (rHRsbleedingvsMI 1.10, 95% CI: 0.82-1.24; I2=16%) (Table 2).
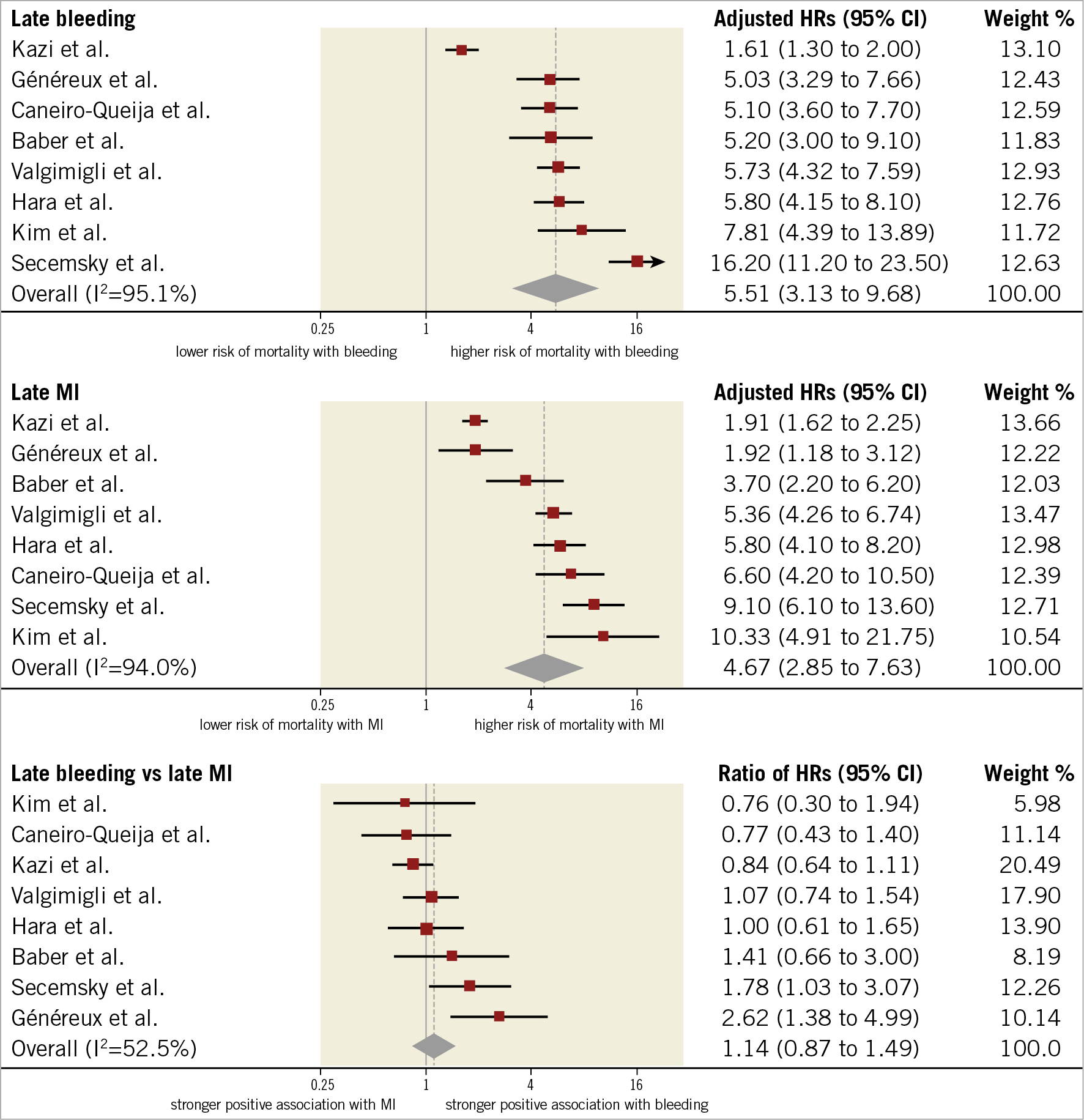
Figure 4. Adjusted hazard ratios for all-cause mortality for patients with late events. HRs for bleeding are for major bleeding.
MINOR BLEEDING vs MI
The median rate of minor late bleeding was 4.7% (IQR 3%-7.1%; 1,523 events/33,597 participants; 4 studies) and the median risk of death among participants with minor late bleeding was 6.3% (IQR 4.6%-8.4%; 73 deaths/1,164 participants; 3 studies). As displayed in Figure 5, the risk of mortality for participants with late minor bleeding (HR 2.11, 95% CI: 1.53-2.90; I2=43%) was lower than for those experiencing late MI (HR 5.78, 95% CI: 4.18-7.99; I2=65%), resulting in a significant rHRs (rHRsbleedingvsMI 0.35, 95% CI: 0.25-0.49, p<0.001; I2=19%). For the only two studies providing data on the composite of late or early minor bleeding, the rHRs was 0.62 (95% CI: 0.42-0.92).
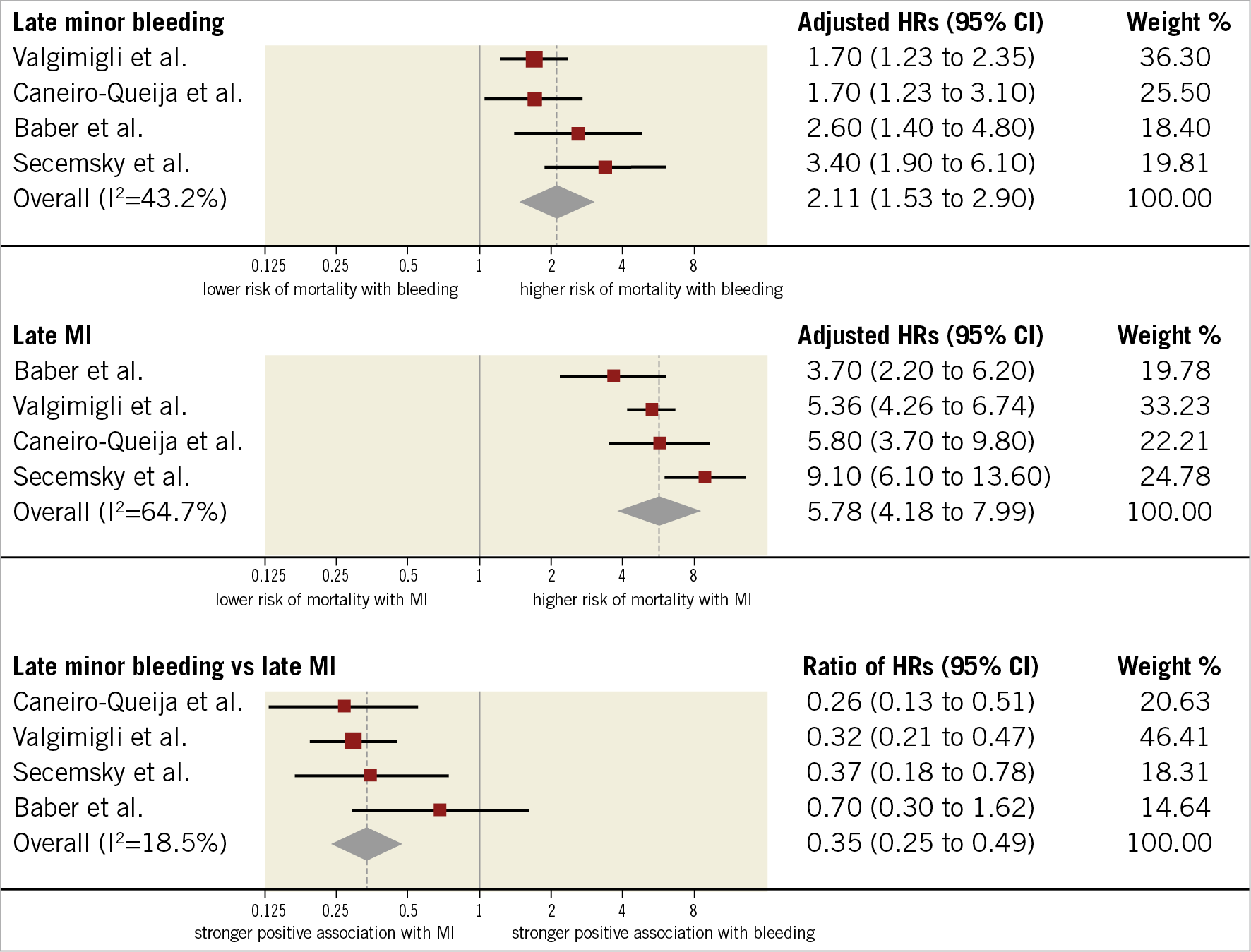
Figure 5. Adjusted hazard ratios for all-cause mortality for patients with late minor bleeding. HRs for bleeding are for minor bleeding.
SENSITIVITY ANALYSES, QUALITY OF STUDIES AND RISK OF BIAS
As shown in Table 2, rHRs for all-cause mortality associated with bleeding versus MI were largely consistent after the exclusion of outlying studies, identified by visual inspection of Galbraith plots (Supplementary Figure 1-Supplementary Figure 24). Results were also consistent between studies published before versus after 2015, the median publication year (Supplementary Table 8). Randomised and non-randomised studies yielded consistent findings, although there was a significant interaction for early events due to a lesser impact of early MI on mortality in non-randomised studies (Supplementary Table 9). All included studies were of high quality according to the Newcastle-Ottawa scale (Supplementary Table 10).
Discussion
In the present systematic review and meta-analysis of 16 studies, including 141,059 patients with CAD, we found strong evidence for a heightened risk of mortality associated with bleeding and MI events. However, there was a differential effect in the relative association between bleeding and MI according to the timing and severity of events: 1) early bleeding had a greater impact on mortality than early MI; 2) the risk of mortality after late bleeding was comparable to the risk of mortality after late MI; 3) the risk of mortality associated with bleeding and MI was comparable for major bleeding, whereas minor bleeding was prognostically less relevant than MI.
Bleeding after PCI is increasingly considered as important as MI. The trend in recent trials investigating antithrombotic therapies after PCI/ACS is to power for superiority with respect to bleeding reduction and only non-inferiority with respect to ischaemic/thrombotic endpoints. However, bleeding continues to represent the most common non-cardiac adverse event after PCI and is associated with increased morbidity and mortality, prolonged hospitalisation, and incremental costs26. Among bleeding avoidance strategies, the choice of vascular access plays a critical role. Indeed, in randomised and large observational studies, radial compared with femoral access showed a strong reduction in the risk of vascular and bleeding complications, affording a parallel decrease by 20-30% in the relative risk of all-cause mortality27,28. In a nested case-control analysis of the MATRIX trial comparing patients who died from causes other than bleeding with 1,370 matched control patients, actionable bleeding was associated with a threefold increase in the risk of mortality, therefore suggesting a link between bleeding prevention and mortality benefit related to radial access29. Similarly, in a trial of patients with chronic CAD and atrial fibrillation, a less intense antithrombotic regimen with rivaroxaban instead of rivaroxaban plus a single antiplatelet agent was associated with a 60% and 55% relative decrease in the risk of major bleeding and death, respectively30. A variety of mechanisms may help to explain the trade-off between bleeding and ischaemia. Discontinuation of antithrombotic therapy or the use of less effective drugs in patients with bleeding complications may result in an increased risk of ischaemic complications. Anaemia can directly affect oxygen delivery to the myocardium and induce a prothrombotic status by triggering erythropoietin release. Transfusions that are frequently required to correct anaemia in the setting of bleeding have been associated with poorer outcomes31. Also, major bleeding may increase the risk of mortality in patients suffering from trauma or malignancies.
Conversely, interventions proven effective in preventing ischaemic complications at the expense of increased major bleeding have not necessarily translated into a mortality benefit. In a patient-level meta-analysis of 48,817 patients, extended clopidogrel therapy on a background of aspirin significantly reduced the risk of ischaemic events and increased the risk of major bleeding, including fatal events, with no effect on mortality32. Along the same lines, in a meta-analysis of 10 trials, prolongation (≥12 months) of dual antiplatelet therapy after PCI yielded increased risks of major bleeding and all-cause mortality despite reduced risks of MI and stent thrombosis6. This observation, however, was largely influenced by a single randomised trial with a higher risk of non-cardiovascular fatalities among patients randomised to prolonged thienopyridine treatment, principally due to cancer-related deaths in the absence of clinically evident bleeding liability33. Collectively, these results might be reconciled in view of the comparable prognostic impact of late bleeding and MI on mortality as supported by the present study.
Another principal finding of our meta-analysis is that early bleeding showed a greater impact on mortality than early MI. Again, this may explain the mortality benefit observed with radial access that is attributable to the prevention of access-site bleeding and also the decrease in major cardiovascular events reported by trials using safer anticoagulant strategies among patients undergoing PCI34. At the same time, it is noteworthy that early MI presented a weaker association with mortality with a pooled risk estimate similar to minor bleeding (HR 2.45 vs 2.11, respectively).
The present study may also have implications for the design of future randomised clinical trials. Given the equipoise between MI and major bleeding in terms of mortality, a net clinical outcome (or net clinical benefit) might be used as the primary endpoint in order to assess the risk-benefit ratio of a given intervention thoroughly and also increase the total number of endpoint events, resulting in more statistical precision and more accurate trials. Finally, by providing a quantitative description of the mortality risk associated with MI and different types of bleeding, the findings of this study may serve as the basis for the estimation of weighting factors in weighted composite endpoint methodologies, in which non-fatal events are accounted for differently based on their relative severity35.
Study limitations
The results of this study should be interpreted in view of several limitations. First, bleeding and MI definitions were different across studies and this represented a source of heterogeneity, which unfortunately was present for most of the outcomes. Moreover, early events included both periprocedural and within 30-day post-PCI events. Similarly, late events included both spontaneous and after 30-day post-PCI events. However, associations remained largely unchanged after the exclusion of outlying studies. Second, although the meta-analysis showed an equipoise between major bleeding and MI in terms of subsequent risk of mortality, our findings do not prove causation of this association. Third, follow-up duration and periprocedural management, including antithrombotic therapy and vascular access, were not uniform across studies. Fourth, the included studies were published over a long period (from 2008 to 2020). However, the results remained largely consistent between newer versus older studies. Fifth, not all the included studies contributed to the analyses which might have affected the precision of pooled risk estimates. Sixth, although all studies had to provide HRs adjusted at least for age, there was substantial variation in the covariates used. Seventh, we used Galbraith plots to identify outlying studies visually, although they have limited value when the number of studies is low.
Conclusions
Our analysis showed that major and late bleedings were associated with a similar increase in the risk of all-cause mortality compared with MI. In the setting of PCI, early bleeding might have a stronger association with mortality than MI, emphasising the importance of bleeding avoidance strategies in perioperative management of patients undergoing percutaneous myocardial revascularisation.
|
Impact on daily practice Major and late bleedings should be considered prognostically equivalent to MI, given the similar association with mortality. Early bleeding has an even stronger association with mortality than early MI, emphasising the importance of bleeding avoidance strategies among patients undergoing PCI. |
Conflict of interest statement
R. Piccolo reports personal fees from Abbott Vascular. S. Windecker reports research and educational grants to the institution from Abbott, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson&Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, and Sinomed. M. Valgimigli reports grants and personal fees from Terumo, personal fees from AstraZeneca, Alvimedica/CID, Abbott Vascular, Daiichi Sankyo, Opsens, Bayer, CoreFlow, Idorsia Pharmaceuticals Ltd, Universität Basel Dept. Klinische Forschung, Vifor, Bristol Myers Squibb SA, iVascular, and Medscape. P. Jüni serves as unpaid member of the steering group of trials funded by AstraZeneca, Biotronik, Biosensors, St. Jude Medical and The Medicines Company, has received research grants to the institution from AstraZeneca, Biotronik, Biosensors International, Eli Lilly and The Medicines Company, and honoraria to the institution for participation in advisory boards and/or consulting from Amgen, Ava and Fresenius, but has not received personal payments by any pharmaceutical company or device manufacturer, and he has no other relationships or activities that could appear to have influenced the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

