Abstract
Aims: The aim of this study was to assess the impact of bleeding after percutaneous coronary intervention (PCI) on the outcome of patients with type 2 diabetes.
Methods and results: This study included 4,329 diabetic patients who underwent PCI. Bleeding was assessed using the Bleeding Academic Research Consortium criteria. The primary outcome was one-year mortality. Bleeding events occurred in 474 patients (10.9%). Access-site and non-access-site bleeds occurred in 274 patients (58%) and 200 patients (42%), respectively. Within the first year after PCI there were 198 deaths: 45 deaths (9.6%) among patients with bleeding and 153 deaths (4.0%) among patients without bleeding (adjusted hazard ratio=2.04 [95% confidence interval 1.38–3.00], p<0.001). There were 25 deaths (12.7%) among patients with non-access-site bleeding and 20 deaths (7.4%) among patients with access-site bleeding (odds ratio [OR]=3.45 [2.20-5.41], p<0.001 for non-access-site bleeding vs. no bleeding and OR=1.90 [1.17-3.01], p=0.008 for access-site bleeding vs. no bleeding). Bleeding improved the discriminatory power of the multivariable model for mortality prediction (p=0.002).
Conclusions: In patients with diabetes undergoing PCI, occurrence of bleeding within the first 30 days after PCI was associated with a significant increase in the risk of one-year mortality.
Introduction
Diabetes mellitus has reached epidemic proportions and its prevalence is trending upwards with estimates allowing the prediction of an increase in prevalence up to 366 million (4.4%) by 20301. The prevalence of coronary heart disease is as high as 55% among adult patients with diabetes2, and cardiovascular events are responsible for three-quarters of hospitalisations and up to 80% of deaths in these patients3. One-quarter to one-third of all coronary revascularisations are performed in diabetic patients4,5. Even in the current era of percutaneous coronary interventions (PCI) and antithrombotic therapy, the risk of death, myocardial infarction and repeat revascularisation remains higher in diabetic patients than in those without diabetes6,7. However, the exact reasons for poor outcome and excess mortality in diabetic patients after PCI remain unknown8. Ample evidence exists that bleeding occurring in the setting of PCI is associated with an increased risk of short and long-term mortality compared with patients who do not bleed9-12. However, the impact of peri-PCI bleeding complications in patients with diabetes has not been studied. We undertook this study to investigate the impact of bleeding on one-year clinical outcome in patients with type 2 diabetes undergoing PCI.
Methods
PATIENTS
The source sample comprised 14,180 patients recruited in seven randomised trials13-19 between June 2000 and May 2011: the ISAR-REACT (Intracoronary Stenting and Antithrombotic Regimen-Rapid Early Action for Coronary Treatment) trial (441 patients with diabetes), the ISAR-SWEET (Intracoronary Stenting and Antithrombotic Regimen: Is Abciximab a Superior Way to Eliminate Elevated Thrombotic Risk in Diabetics) trial (701 patients with diabetes), the ISAR-SMART-2 (Intracoronary Stenting to Abrogate Restenosis in Small Arteries) trial (139 patients with diabetes), the ISAR-REACT 2 trial (536 patients with diabetes), the ISAR-REACT 3 trial (1,254 patients with diabetes), the ISAR-REACT 3A trial (758 patients with diabetes) and the ISAR-REACT 4 trial (500 patients with diabetes). Thus, in all seven trials, there were 4,329 patients with type 2 diabetes who were included in this study. Detailed inclusion/exclusion criteria were provided in the primary publications13-19.
Written informed consent was required for participation, and an institutional review board approved each study at the respective participating centres. The study conforms to the Declaration of Helsinki.
PCI AND PERIPROCEDURAL ANTITHROMBOTIC DRUGS
Coronary stenting was performed according to standard practice. Coronary stents were implanted in 3,935 patients (91%). Drug-eluting stents were used in 2,548 patients (64.7% of stents). The femoral artery route was used for vascular access. Sheaths were removed and manual compression was applied as soon as the activated partial thromboplastin time fell below 50 seconds. Vascular access closure devices were used in <10% of the patients. Before PCI, patients received 325-500 mg of aspirin and a 600 mg loading dose of clopidogrel. Four antithrombotic drug regimens were used: abciximab as a bolus of 0.25 mg/kg weight followed by an infusion of 0.125 µg/kg/min (a maximum of 10 µg/min) for 12 hours in combination with a 70 U/kg intravenous bolus of unfractionated heparin; unfractionated heparin as a 140 U/kg intravenous bolus; unfractionated heparin as a 100 U/kg intravenous bolus; and bivalirudin as a bolus of 0.75 mg per kilogram, followed by an infusion of 1.75 mg per kilogram per hour for the duration of the procedure.
Chronic post-stenting antithrombotic therapy consisted of aspirin (80-325 mg per day indefinitely), clopidogrel (75-150 mg per day until discharge but for no longer than three days, followed by 75 mg per day for at least one month, in patients with bare metal stents, or six months in patients with drug-eluting stents). Other cardiac medications were prescribed at the discretion of the patient’s physician.
DEFINITIONS AND OUTCOMES
Cardiovascular risk factors were defined using accepted criteria. Type 2 diabetes was diagnosed based on: history of diabetes with active treatment with insulin or oral hypoglycaemic agents on admission; documentation of an abnormal fasting blood glucose (≥7.0 mmol/l) or glucose tolerance test (≥11.1 mmol/l) according to World Health Organization criteria for diabetes; or a blood glucose >200 mg/dl at any time. Creatinine clearance was calculated using the Cockcroft-Gault equation20.
Bleeding events within the 30 days after PCI were defined using the Bleeding Academic Research Consortium (BARC) criteria21. BARC criteria were retrospectively applied. For every bleeding event, source documentation was available and was re-assessed. Data on the site of bleeding, haemoglobin level, haematocrit, imaging tests and blood transfusion were re-analysed. Since the primary outcome of this analysis was mortality, bleeding events potentially belonging to BARC class 5 (fatal bleeding) were distributed to other classes according to their initial characterisation. Access-site bleeding was defined as bleeding or haematoma arising from the arterial access site area including bleeding that spread to adjacent tissues (such as the retroperitoneal space). Non-access-site bleeding was defined as bleeding not arising from the arterial access site. Only one bleeding event per patient was analysed. In the case of more than one bleeding event, the location of bleeding was defined as the site with the largest bleed. Bleeding of undefined location after inconclusive imaging tests (including imaging for retroperitoneal haematoma) was considered as non-access-site bleeding.
The primary outcome was one-year mortality. Non-fatal myocardial infarction and stent thrombosis within the first year after PCI were also assessed. Information on deaths was obtained from hospital records, death certificates, or telephone contact with relatives of the patient or referring physician. The diagnosis of myocardial infarction required the development of new abnormal Q-waves in ≥2 contiguous precordial or ≥2 adjacent limb leads or an elevation of creatine kinase-MB >2 times (>3 times for the 48 hours after a PCI procedure) the upper limit of normal. Definite stent thrombosis was defined according to the Academic Research Consortium criteria22. Adjudication and classification of the bleeding events were performed by personnel not involved in the primary studies. Patients were either seen by their physician or interviewed by telephone at 30 days, six months, and one year after the procedure. In case of documented or suspected cardiac complaints, patients underwent a complete clinical, ECG, and laboratory evaluation any time during the follow-up.
STATISTICAL ANALYSIS
Data are presented as median (25th, 75th percentiles), proportions (%) or Kaplan-Meier estimates (%). The Kolmogorov-Smirnov test was used to assess the normality of data distribution. Continuous data were compared with the Kruskal-Wallis rank-sum test. Categorical data were compared with the chi-square test. A multiple logistic regression model was used to assess independent associates of bleeding. All variables in Table 1 with the exception of the type of antithrombotic therapy were entered into the model. The association of antithrombotic therapy with bleeding was assessed based on randomised comparisons as used in the primary randomised studies. The survival analysis was performed using the Kaplan-Meier method and the log-rank test. A multivariable Cox proportional hazards model was used to assess the association between bleeding and one-year mortality, myocardial infarction or stent thrombosis. All variables in Table 1 plus bleeding were entered into the model. To avoid co-linearity with bleeding, type of antithrombotic therapy was not entered into the Cox model. The proportional hazards assumption was checked using the method of Grambsch and Therneau23. Discriminatory power of the Cox model(s) before (with baseline variables only) and after inclusion of bleeding was assessed by calculating the integrated discrimination improvement (IDI)24. All analyses were performed using the R package. A two-sided p-value <0.05 was considered to indicate statistical significance.
Results
PATIENT CHARACTERISTICS
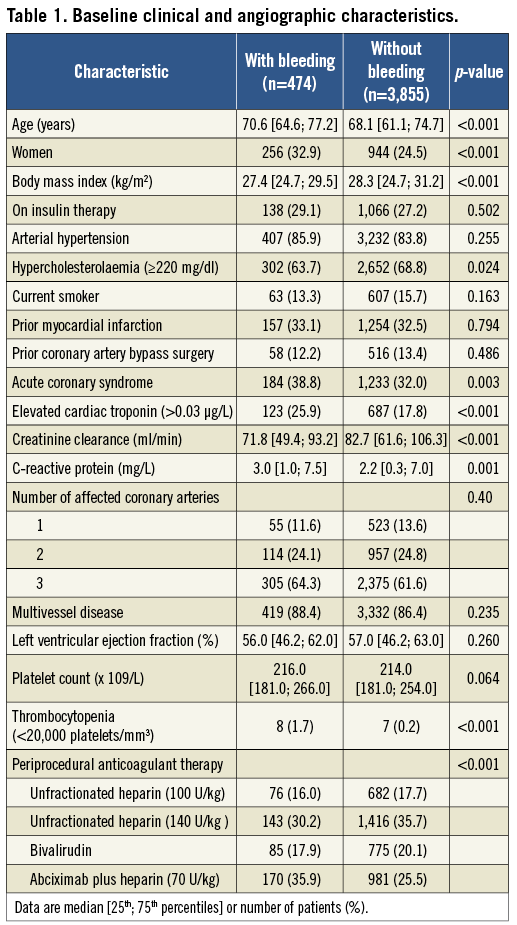
The study included 4,329 patients with type 2 diabetes. Within 30 days after PCI, bleeding events occurred in 474 patients (10.9%). Baseline characteristics of patients with and without bleeding are shown in Table 1. Patients who bled were older and more likely to be women, had lower body mass index and hypercholesterolaemia, presented more often with an acute coronary syndrome and elevated troponin, and had lower creatinine clearance and higher C-reactive protein levels. Thrombocytopenia was more frequent among patients who bled. There appeared to be differences among bleeders and non-bleeders regarding the type of antithrombotic therapy (Table 1). Coronary stents were implanted in 422 patients with bleeding and in 3,513 patients without bleeding (89.0% vs. 91.2%; p=0.297). Drug-eluting stents were implanted in 275 patients with bleeding and in 2,273 patients without bleeding (58.0% vs. 59.0%; p=0.693).
BLEEDING EVENTS
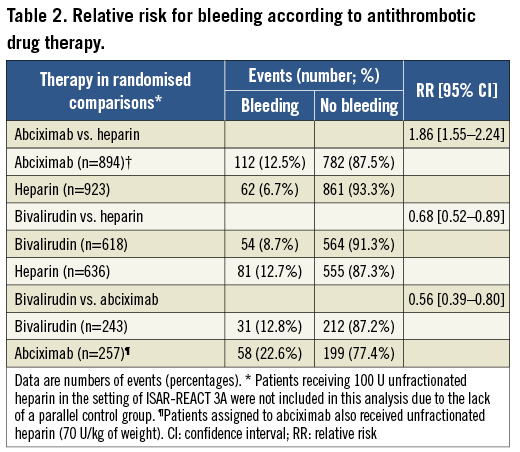
Severity of bleeding according to BARC criteria was as follows: class 1 (180 patients; 38.0%), class 2 (69 patients; 14.6%), class 3a (165 patients; 34.8%), class 3b (55 patients; 11.6%), class 3c (three patients; 0.6%) and class 4 (two patients; 0.4%). Bleeding of class ≥2 occurred in 294 patients (6.8%). Of 474 bleeds, 274 (58%) were access-site bleeds and 200 (42%) were non-access-site bleeds. Multiple logistic regression (see Methods for variables entered into the model) identified three independent correlates of the increased risk for bleeding: body mass index (adjusted odds ratio [OR]=0.78, 95% confidence interval [CI] 0.67-0.91; p=0.001 for each 5 kg/m2 increase), reduced renal function (adjusted OR=1.22 [1.04-1.43]; p=0.016 for each 30 ml/min decrease in the creatinine clearance) and elevated troponin (adjusted OR=1.46 [1.00-2.15]; p=0.050 compared with patients without elevated troponin). Due to differences in the baseline characteristics across randomised trials, the assessment of the role of antithrombotic therapy on the occurrence of bleeding was confined to randomised comparisons as used in the primary randomised studies (Table 2). As seen in Table 2, bivalirudin significantly reduced the frequency of bleeding compared to abciximab or unfractionated heparin, leading to a 44% and 32% reduction in the risk of bleeding, respectively. The use of unfractionated heparin (140 U/kg of weight) was also associated with a significant reduction in the risk of bleeding compared with combined therapy of abciximab plus unfractionated heparin (70 U/kg of weight).
CLINICAL OUTCOME
Overall, there were 198 deaths within the first year after PCI: 45 deaths among patients who bled and 153 deaths among those who did not bleed (Kaplan-Meier estimates 9.6% and 4.0%, respectively, OR=2.51 [1.82-3.47], p<0.001, Figure 1). There were 25 deaths among patients with non-access-site bleeding and 20 deaths among patients with access-site bleeding (Kaplan-Meier estimates 12.7% and 7.4%, respectively; OR=3.45 [2.20-5.41], p<0.001 for non-access-site bleeding vs. no bleeding; OR=1.90 [1.17-3.01], p=0.008 for access-site bleeding vs. no bleeding and OR=1.80 [1.01-3.21], p=0.047 for non-access-site bleeding vs. access-site bleeding; Figure 2).
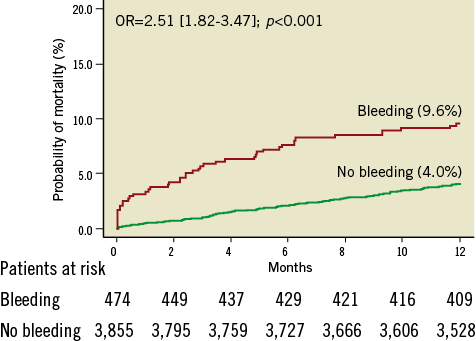
Figure 1. Kaplan-Meier curves of one-year mortality in diabetic patients with and without bleeding. Numbers (%) show Kaplan-Meier estimates of one-year mortality. OR: odds ratio
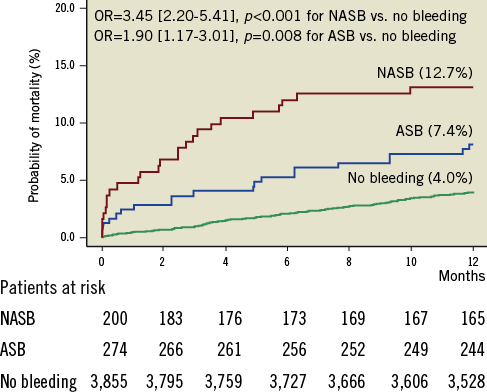
Figure 2. Kaplan-Meier curves of one-year mortality in diabetic patients with non-access-site (NASB), access-site (ASB) and no bleeding. Numbers (%) show Kaplan-Meier estimates of one-year mortality. OR: odds ratio
Non-fatal myocardial infarction within the first year after PCI occurred in 276 patients: 70 infarctions occurred among patients who bled and 206 infarctions occurred among those who did not bleed (Kaplan-Meier estimates 14.8% and 5.4%, respectively, OR=2.95 [2.28-3.83], p<0.001). There were 29 infarctions among patients with non-access-site bleeding and 41 infarctions among patients with access-site bleeding (Kaplan-Meier estimates 14.7% and 15.0%; OR=3.00 [1.98-4.56], p<0.001 for non-access-site bleeding vs. no bleeding and OR=3.11 [2.17-4.67], p<0.001 for access-site bleeding vs. no bleeding). There appears to be no significant difference in the frequency of myocardial infarction between non-access-site bleeding and access-site bleeding (OR=0.97 [0.60-1.56], p=0.902).
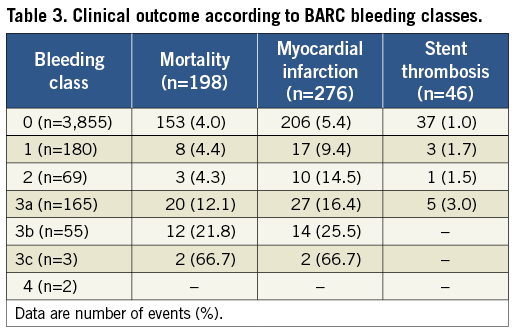
Definite stent thrombosis occurred in 46 patients: nine thromboses among patients who bled and 37 thromboses among those who did not bleed (Kaplan-Meier estimates 2.0% and 1.0%; OR=2.04, [1.00-4.16], p=0.05). Clinical outcome according to BARC bleeding class is shown in Table 3.
RESULTS OF MULTIVARIABLE ANALYSIS
The Cox proportional hazards model was used to test the association between bleeding and mortality, myocardial infarction or stent thrombosis, while adjusting for other variables (see Methods for variables entered into the model). The model showed that bleeding was an independent correlate of one-year mortality (adjusted hazard ratio [HR]=2.04 [1.38-3.00], p<0.001), myocardial infarction (adjusted HR=2.89 [2.13–3.90], p<0.001) and stent thrombosis (adjusted HR=2.57 [1.16–5.70], p=0.020).
The impact of bleeding on the discriminatory power of the multivariable model regarding mortality, myocardial infarction or stent thrombosis was assessed by calculating the IDI. The inclusion of bleeding in the Cox model improved the discriminatory power of the model for prediction of mortality (absolute and relative IDI, 0.0098 and 16.3%, respectively, p=0.002), myocardial infarction (absolute and relative IDI, 0.016 and 55.1%, respectively, p<0.001) but not stent thrombosis (absolute and relative IDI, 0.0027 and 23.1%, respectively, p=0.248).
Discussion
The main findings of the present study are as follows. 1) In patients with type 2 diabetes, the occurrence of bleeding within the first 30 days after a PCI procedure was associated with a significant increase in the risk of death, non-fatal myocardial infarction or stent thrombosis. Of note, the occurrence of bleeding doubled the risk of mortality and stent thrombosis and almost tripled the risk of non-fatal myocardial infarction. 2) A small body mass index, reduced renal function, elevated troponin and type of antithrombotic therapy were associated with an increased risk of bleeding. The use of bivalirudin therapy significantly reduced the risk of bleeding compared with the glycoprotein IIb/IIIa receptor inhibitor abciximab or unfractionated heparin. 3) Bleeding improved the discriminatory power of multivariable models for risk prediction denoting that peri-PCI bleeding provides prognostic information that is independent of and beyond that provided by cardiovascular risk factors and relevant clinical variables.
A high baseline bleeding risk in diabetic patients has been reported and explained by increased vascular fragility in these patients5,10,25. An analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial, which included patients with an acute coronary syndrome, showed a strong trend (a 20% increase in the adjusted risk) for an independent association between diabetes and major bleeding10. A report from the National Cardiovascular Data Registry (2004 to 2008) showed that diabetes was present in 1/3 of older US patients undergoing PCI in the drug-eluting stent era. Of note, over a median of 18.4 months of follow-up, diabetes was associated with a 39% relative increase in the hazard of hospitalisation for bleeding compared with non-diabetic patients5. In the present study, bleeding events were found in 10.9% of the patients. Of note, serious bleeding (BARC class ≥2) occurred in 6.8% of the patients. Comparison of the present findings with prior studies is difficult due to the use of various bleeding definition criteria with substantial differences in their sensitivity to identify relevant bleeding events. However, a frequency of BARC class ≥2 of 6.8%, as found in the present study in diabetic patients, is higher than the frequency of BARC class ≥2 of 5.4% in a mixed group of non-diabetic and diabetic patients treated with PCI26.
In the present study, we found that the occurrence of bleeding was associated with excess mortality and an increased risk of ischaemic/thrombotic events such as myocardial infarction and stent thrombosis. Although the reasons for the excess mortality and myocardial infarction or stent thrombosis in diabetic patients who bled after PCI are not entirely known, some explanations may be offered. The deleterious effects of bleeding may be the same as in patients without diabetes27-29. However, diabetes and bleeding complications may act synergistically in promoting an increased risk of death or acute thrombotic events (myocardial infarction and stent thrombosis) for at least two reasons: first, combination of bleeding and diabetes – each known to be associated with various comorbidities – may lead to a markedly worse cardiovascular risk profile in those with both conditions; and second, both diabetic status30 and bleeding27,28 may accentuate a prothrombotic state leading to an increased frequency of ischaemic/thrombotic events in patients with both conditions. As for the higher risk of mortality associated with non-access-site bleeding compared with access-site bleeding, this finding has been described in patients undergoing PCI irrespective of diabetic status31 and, as such, it is not specific or restricted to patients with diabetes.
Limitations
The present study has some limitations. First, vascular access for PCI was achieved by the femoral artery route and thus the study findings may not be extrapolated to patients undergoing PCI via the radial artery route, known to reduce peri-PCI bleeding. Second, BARC bleeding criteria were applied retrospectively. Although bleeding data were collected as part of prospective randomised trials and detailed source documentation was available and was re-assessed, we acknowledge the possibility of having missed some mild forms of bleeding belonging to BARC class 1, particularly if they occurred after discharge to 30 days. Third, data on metabolic control of patients were not available in all patients, so any impact of this factor on outcome remains unexplored. Fourth, this pooled analysis included various antithrombotic regimens with differences in their bleeding risk. Finally, the number of stent thromboses was too small to allow a more detailed analysis of predisposing factors for this adverse event.
Conclusions
In conclusion, peri-PCI bleeding in patients with diabetes is associated with an increased risk of one-year mortality, non-fatal myocardial infarction or stent thrombosis. Peri-PCI bleeding provides prognostic information that is independent of and beyond that provided by cardiovascular risk factors and relevant clinical variables. These data suggest that bleeding avoidance strategies should preferentially be used in diabetic patients to reduce the occurrence of bleeding and its deleterious impact on mortality and morbidity.
| Impact on daily practice Bleeding complications among patients with type 2 diabetes undergoing percutaneous coronary interventions are frequent and they increase the risk of one-year mortality, myocardial infarction and stent thrombosis. The propensity for periprocedural bleeding was high among diabetic patients with small body mass, reduced renal function, elevated troponin and among those who received glycoprotein IIb/IIIa receptor inhibitor abciximab as adjunct periprocedural antithrombotic therapy. The use of bivalirudin as anticoagulant/antithrombotic therapy reduced the risk of periprocedural bleeding compared with unfractionated heparin alone or combined with abciximab. Bleeding-avoidance strategies may be preferentially used in diabetic patients undergoing percutaneous coronary interventions to reduce the frequency of bleeding and minimise its deleterious impact on mortality and morbidity. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

