Abstract
Aims: While bleeding in patients with STEMI undergoing primary percutaneous coronary intervention (pPCI) is known to be associated with poor outcomes, the differential prognostic impact of access-site related versus non access-site related bleedings is unknown. We aimed to assess the relative impact of access-site related bleeding, as compared to non access-site related, on 12-month clinical outcome in patients undergoing intervention for STEMI.
Methods and results: Thirty-day bleeding endpoints, stratified into access-site versus non access-site, were examined according to the TIMI scale in 744 patients with STEMI enrolled in the MULTISTRATEGY trial. TIMI major or minor bleeding complications occurred in 56 (7.5%) patients within 30 days, 46% had an access-site related bleed and 34% required blood transfusion. Bleeding severity and the need for transfusion were equally distributed between site access- versus non-site access-related bleeds. After adjustment, patients with any TIMI rated bleed were more likely to die or develop recurrent MI within 12 months (HR 2.1 [95% CI: 1.13-3.8]; p=0.02). This ratio was entirely driven by non-site access-related bleeds (adjusted HR: 2.66 [95% CI: 1.21-5.8]; p=0.007), whereas site-access bleeds were not associated with worse outcomes (HR: 0.74 [95% CI: 0.16-3.4]; p=0.70).
Conclusions: While bleeds of any TIMI severity within 30 days were independently associated with worse cardiovascular outcomes at 12 months, thus confirming previous analyses, this relationship was entirely driven in our study by non access-site related haemorrhagic events. Investigation on whether the site of bleeding complications may preferentially impact cardiovascular outcomes is warranted.
Introduction
The combined use of anticoagulants, potent anti-platelet agents (e.g., glycoprotein IIb/IIIa [Gp IIb/IIIa] receptor inhibitors)1-7 and an invasive strategy in patients with STEMI reduces ischaemic coronary events but also increases bleeding8-9.
The Janus (Roman god of gates and doors, of beginnings and endings, represented as a double-faced head looking in opposite directions) nature of refined concomitant antithrombotic therapies may reveal itself under highly (pro−) thrombogenic conditions, such as contemporary pPCI. On the one hand, periprocedural and deferred (athero-) thrombotic events are a real concern while stenting in a vessel with visible thrombus, especially when using active stents10. On the other hand, we face bleeding and its attendant risks of death and MI8,11.
There is a paucity of data on whether the site of bleeding, access-site related or not, affects cardiovascular outcomes after PCI. This information has relevant clinical and pathophysiological implications. Although not all bleeding can be directly attributed to complications at the procedure access-site, the latter remain a significant factor in post-procedural bleeding8. They can largely be prevented by technical refinements during puncture or implementing a routine radial approach. Non access-site bleeding complications mainly reflect co-morbidity and intensity/duration of anti-thrombotic therapy. Thus, strategies to prevent bleeding complications, targeting different pathophysiological components, may have different impacts on preventing access-site versus non access-site bleeding events.
As part of a pre-specified sub-analysis of the MULTISTRATEGY trial, we investigated the relative impact of access-site related bleeding, as compared to non access-site related, on one-year clinical outcome in patients undergoing pPCI.
The rationale, design and methodology of the MULTISTRATEGY study have been detailed in a previous publication and are outlined below12,13. Briefly, patients were randomly assigned with the use of a two-by-two factorial design to one of four interventional strategies of reperfusion: abciximab with an uncoated stent, abciximab with a sirolimus-eluting stent, tirofiban with an uncoated stent, or tirofiban with a sirolimus-eluting stent. The study protocol, including this pre-specified “bleeding” sub-analysis, was approved by the ethics committee at each institution and was conducted according to the principles of the Declaration of Helsinki. All patients provided written informed consent before enrolment. Follow-up visits were scheduled at one month, four months, eight months and 12 months.
Study population and study protocol
Patient population
Patients in the MULTISTRATEGY study were enrolled at 16 centres in Italy, Argentina and Spain. Patients who entered MULTISTRATEGY had to be ≥18 years of age, be admitted for 1: chest pain for longer than 30 minutes with either an electrocardiographic ST-segment elevation of 1 mm or greater in two or more contiguous electrocardiogram leads or with a new left bundle-branch block, and 2: admission either within 12 hours of symptom onset or between 12 and 24 hours after onset with evidence of continuing ischaemia. The exclusion criteria included administration of thrombolysis in the previous 30 days, major surgery within 15 days, and active bleeding or previous stroke in the last six months.
Concomitant anticoagulation treatment
Immediately after checking eligibility criteria and before the visualisation of coronary angiographic anatomy, the local site investigators performed open-label assignments of study treatments via sealed envelopes. Randomisation was achieved with a 1:1:1:1 computer-generated random sequence supplied by an academic statistician, without stratification, in permuted blocks of 30. Either tirofiban or abciximab was administered at first medical contact before an arterial sheath insertion during the angiography procedure. Tirofiban was administered as a bolus of 25 µg/kg, followed by an 18- to 24-hour infusion at 0.15 µg/kg/min. Abciximab was administered as a bolus of 0.25 mg/kg, followed by a 12-hour infusion at 0.125 µg/kg/min. Unfractionated heparin (UFH) was administered at 40 to 70 U/kg, targeting an activated clotting time of at least 200 seconds. All patients received aspirin (160-325 mg orally or 250 mg intravenously, followed by 80-125 mg/d orally indefinitely) and clopidogrel (300 mg orally and then 75 mg/d for at least three months). Stenting was the default strategy in all patients with a reference vessel diameter of 2.5 mm or larger at visual estimation.
Data collection and management
Clinical data were prospectively collected at each site by research nurses or treating physicians using a standardised case report form (CRF). Demographic and clinical characteristics, treatment practices, and hospital outcome data, including information on occurrence and timing of major bleeding, were collected. Medications and procedures received in hospital were categorised into those received during the “first 48 hours of admission (periprocedural)” or those received “anytime during hospitalisation.” Independent study monitors employed by the University of Ferrara verified 100% of the data in the CRFs. End points, including bleeding events, were adjudicated and classified by a blinded clinical events committee (CEC). Independent adjudication of an event trigger, including bleeding, was performed separately by two CEC members; in case of disagreement, the opinion of a third member was obtained, and the final decision taken by consensus.
Standardised definitions of all patient-related variables, clinical diagnoses, selected hospital complications, and outcomes were used2. In-hospital bleedings were graded according to the TIMI trials criteria14. A bleeding complication was defined as major if it was intracranial or if clinically overt signs of haemorrhage were associated with a drop in haemoglobin concentration of more than 5.0 g/dl (or, when a haemoglobin value was not available, an absolute drop in the haematocrit of at least 15%). Minor bleeding was defined as a clinically overt haemorrhage (including that seen on imaging) associated with a fall in haemoglobin concentration of 3.0 to 5.0 g/dl (or, when a haemoglobin value was not available, a fall in the haematocrit of 9% to <15%). Patients were classified as having received a transfusion if they received whole blood or packed red cells at any point during their index hospitalisation.
TIMI study definitions take into account blood transfusions, therefore haemoglobin and haematocrit values are adjusted by 1 g/dl or 3%, respectively, for each unit of blood transfused. Therefore, the true change in haemoglobin or haematocrit is calculated as follows: if there has been an intervening transfusion between two blood measurements: ∆haemoglobin (Hgb) = [baseline Hgb – post-transfusion Hgb] + [number of transfused units]; ∆haematocrit (Hct) = [baseline Hct – post-transfusion Hct] + [number of transfused units×3]15. Date and site of each episode of major bleeding were recorded. Bleeding that occurred after coronary artery bypass graft surgery was not included in this analysis.
We defined major life-threatening bleeding as fatal bleeding, intracranial bleeding, intrapericardial bleeding with cardiac tamponade, hypovolaemic shock or severe hypotension due to bleeding and requiring vasoactive drugs or surgery, a decline in the haemoglobin level of 5.0 g/dl or more, or the need for a transfusion of at least four units of red blood cells (RBC).
Statistical analysis
Categorical variables are expressed as percentages, and continuous variables are expressed as mean and standard deviation. Baseline characteristics were compared with χ2 or Fisher exact tests for categorical variables and the t-test for continuous variables.
Kaplan-Meier analysis was used to illustrate 12-month event-free survival for patients with and without combined TIMI major and minor bleeding within 30 days.
We compared unadjusted rates of the composite end point of death and non-fatal recurrent MI among patients with no TIMI “significant” (major/minor: major and minor combined) bleeding and those with TIMI “significant” bleeding severity and separately for the categories of access-site and non access-site related bleeding. To determine the association between access-site and non access-site related bleeding, we constructed separate models for each category. The first model incorporated “access-site related TIMI major/minor bleeds within 30-days” as a time-dependent covariate and used “no access-site related TIMI major/minor bleeds within 30-days” as the reference. The second model incorporated “non access-site related TIMI major/minor bleeds plus unknown (significant drop in haemoglobin without overt bleeding) within 30-days” as a time-dependent covariate and used “no non access-site related TIMI major/minor bleeds plus unknown within 30-days” as the reference.
A Cox proportional hazards model was used to test the association between TIMI major/minor bleeds within 30 days after the PCI and one-year mortality or non-fatal MI while adjusting for confounding demographic and clinical variables. Baseline differences that were not uniformly distributed in patients with and without bleeding complications based on a p-value of 0.1 or less were considered significant and entered into the multivariable model; these differences include gender, diabetes, baseline creatinine clearance, haemoglobin level at presentation, femoral access, intra-aortic balloon pump (IABP) use, KILLIP class greater than one, right coronary artery as target vessel, vessel size (MLD post-PCI) and final TIMI 3 flow, as well as need for transfusion. Age was not separately entered into the model to avoid co-linearity with creatinine clearance, whose formula includes age.
All analyses, carried out based on the intention to treat principle, were performed using STATA, version 9.2 (Stata Corp., College Station, TX, USA).
Results
Of the 744 patients enrolled in the MULTISTRATEGY trial, 565 (76%) patients were male, and 357 (48%) patients were ≥65 years of age. One hundred and eight (15%) patients suffered from diabetes, and fifteen (3%) patients had documented peripheral artery disease. Table 1 shows the baseline and angiographic characteristics of the patients stratified based on the occurrence of a TIMI major/minor bleed within 30 days. Patients who bled were older and more often female, with a higher prevalence of diabetes, and they presented more frequently with clinical signs of heart failure than patients without significant bleeding, despite similar left ventricular ejection fraction at transthoracic echocardiogram. A higher proportion of patients who experienced a TIMI major/minor bleed within 30 days had a femoral access approach and the use of an IABP. Interestingly, the peak ACT values did not differ in patients with than those without bleeding complications. Fifteen (2.0%) patients experienced a TIMI major bleed, whereas 41 (5.5%) patients fulfilled the TIMI minor bleeding criteria, such that 56 (7.5%) patients experienced a TIMI major or minor bleed within 30 days. Twenty-three bleeding events (3.1%) were judged as life-threatening by the investigators and none were intracranial or intra-ocular.
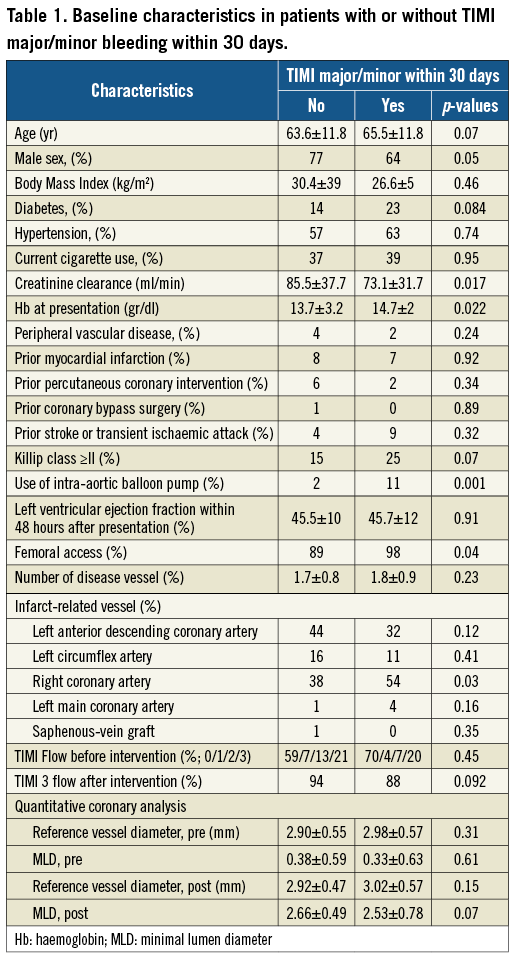
The incidence of bleeding within the various categories of TIMI bleeding severity (major, minor and major-minor combined) did not differ between the Abciximab- and Tirofiban-treated patient groups (Table 2). However, significantly more patients in the Abciximab treatment group had a nadir haemoglobin (Hb) value <8.5 g/dl. This was offset by a slight trend towards more transfusion of at least one unit in the Tirofiban-treated patients; however, equal patient numbers were transfused with >2 units of blood in both treatment arms. Access-site bleeding complications were equally represented in both treatment arms.
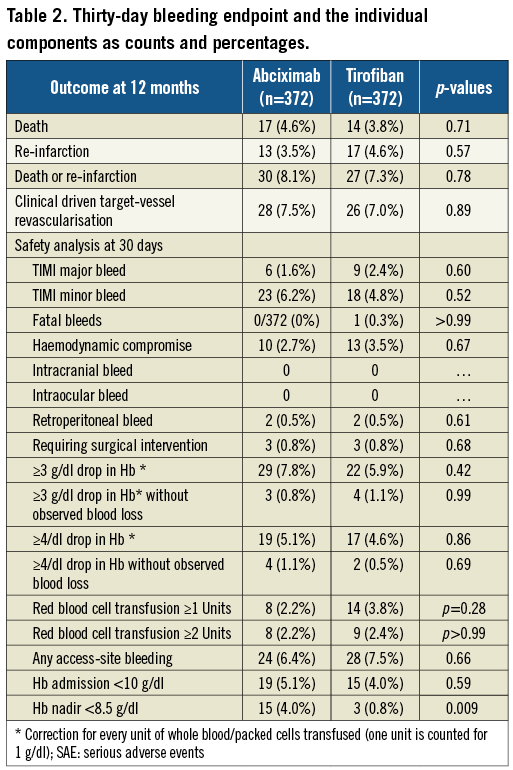
The location of TIMI major/minor bleeding at 30 days was at the site of the vascular access, including retroperitoneal in 26 (46%) patients, gastrointestinal in eight (14%), genitourinary in six (11%), pericardial in three (5%), pulmonary in three (5%), other sites in three (5%) or without overt bleeding site identified in seven (13%). Nineteen out of 56 (34%) patients with TIMI major/minor bleed within 30 days underwent blood transfusion during the hospitalisation; six (11%) patients required surgery to repair or explore the bleeding site. Approximately half the TIMI major/minor bleeds occurred within 48 hours of the pPCI (data not shown). The severity (major versus minor) of TIMI bleeds is shown in Figure 1 and did not differ based on location (site-access versus non site-access).
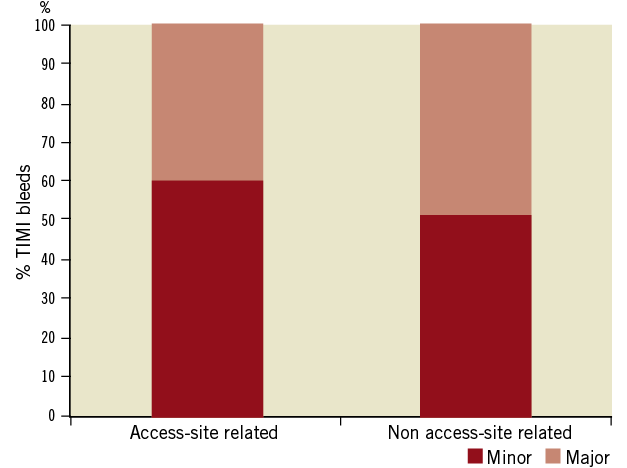
Figure 1. TIMI bleedings stratified, based on site and severity in the MULTISTRATEGY trial. The severity of bleeds according to the TIMI scale did not differ based on the site of haemorrhage.
Impact of bleeds on 12-month outcomes
In the MULTISTRATEGY trial the overall rate of death and the composite of death or myocardial infarction within 12 months was 4.2% and 7.7% respectively.
In the univariate Cox proportional hazards model, patients with TIMI major or minor bleeds within 30 days had more than a two-fold increase in the composite of death or MI at 12 months (HR: 2.45 [95% CI: 1.21-4.97]; p=0.012) (Figure 2). After adjusting for all potential confounders in the multivariable Cox proportional hazards model, including all clinical and angiographic variables that were not uniformly distributed based on bleeding status as reported in Table 2, as well as need for transfusion, TIMI major/minor bleed was still associated with a two-fold increase in the composite of death or MI within 12 months (HR: 2.1 [95% CI: 1.13-3.8]; p=0.02). Similarly, when transfusion was restricted to patients receiving at least two red blood cell units, the multivariable analysis yielded identical results.
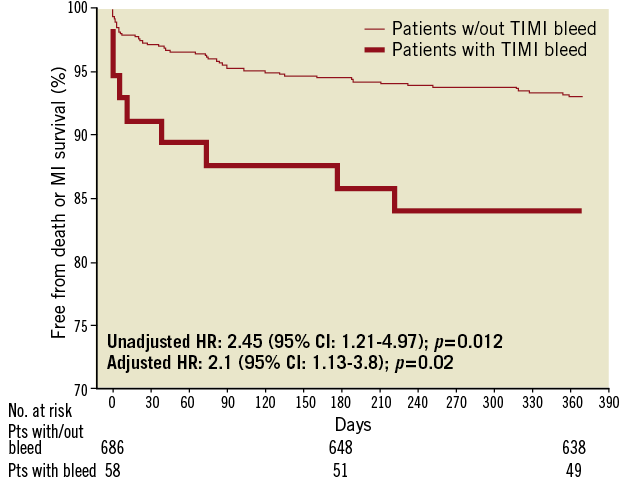
Figure 2. Cumulative Kaplan–Meier estimates of mortality and reinfarction rates during follow-up based on bleeding status. Cumulative risk of death and reinfarction at 370 days in patients with or without TIMI-defined major or minor bleeding complications within 30 days of pPCI. Overall four patients were lost to follow-up between eight and 12 months.
When TIMI major or minor bleeding events were stratified based on their location, access-site bleeding complications were not associated with a worse 12-month outcome (adjusted HR: 0.74 [95% CI: 0.16-3.4]; p=0.70), whereas non access-site related bleeds were associated with a worse 12-month outcome (Figure 3), both after univariate analysis (HR: 4.1 [95% CI: 1.85-8.9]; p<0.001) and multivariable adjustment (HR: 2.66 [95% CI: 1.21-5.8]; p=0.007) (Figure 4; Table 3). Excluding bleeds from unidentified sites yielded identical results. Similarly, adding any transfusion or blood transfusion of at least two blood units failed to influence our model (data not shown).
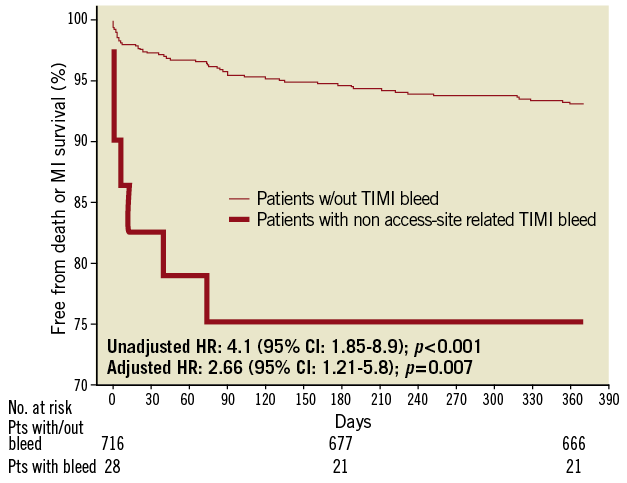
Figure 3. Cumulative Kaplan–Meier estimates of mortality and reinfarction rates during follow-up based on non access-site bleeding status. Cumulative risk of death and reinfarction at 370 days in patients with non-site access-related TIMI major or minor complications or without any bleeds within 30 days of pPCI. Overall four patients were lost to follow-up between eight and 12 months.
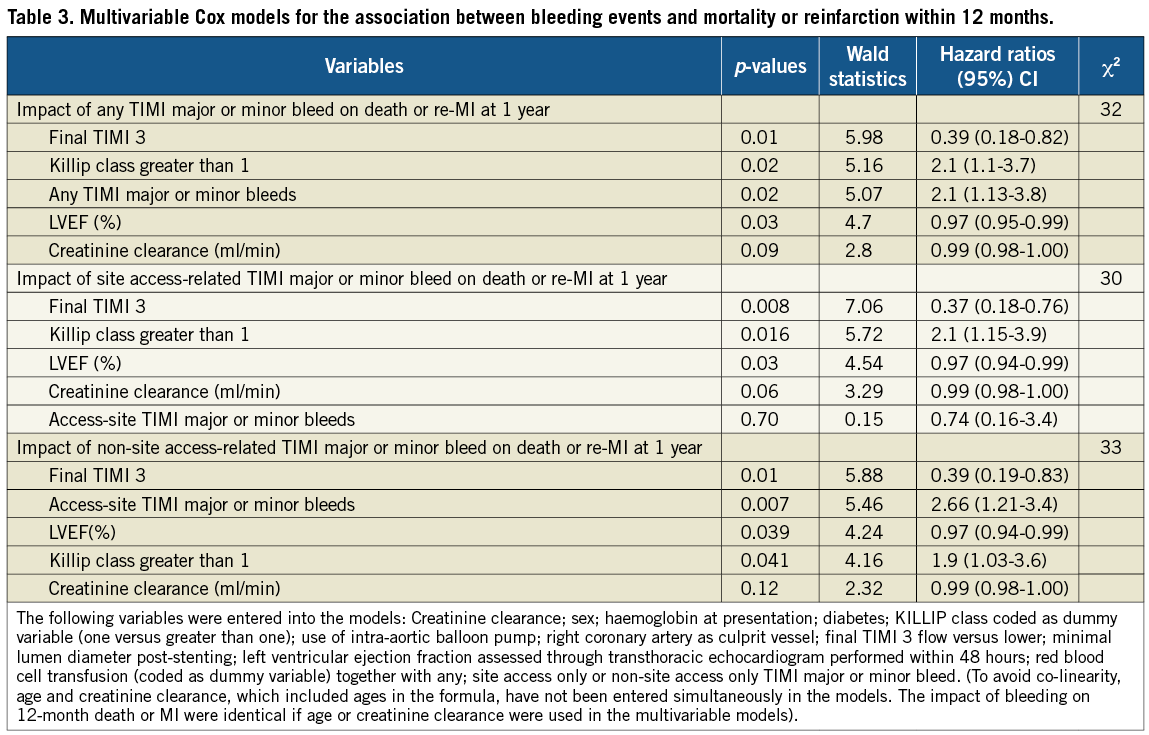
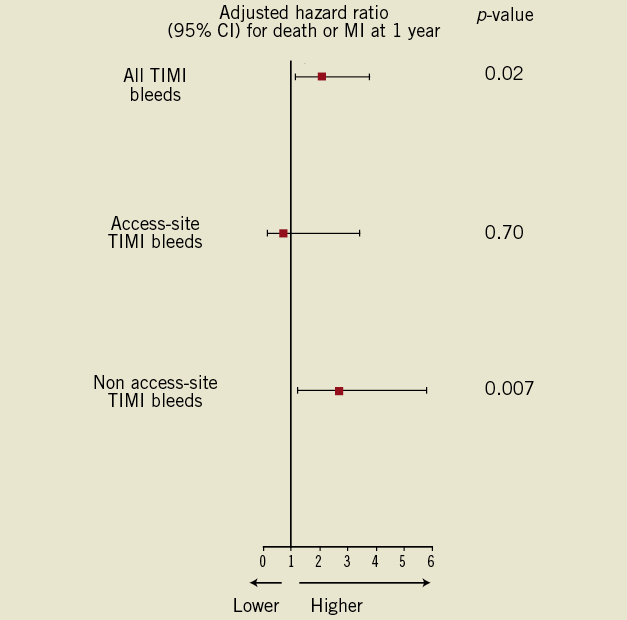
Figure 4. Stratified analysis on the site of bleeding. Adjusted hazard ratios for death or reinfarction within 370 days according to whether any TIMI major/minor bleed (access-site related or non access-site related) occurred within 30 days of pPCI.
Discussion
The analysis of the relationship between bleeds within 30-day and 12-month outcomes can be summarised as follows:
1. TIMI major/minor bleed within 30 days were independently associated with worse cardiovascular outcomes, including overall death and non-fatal MI within 12 months, confirming previous analyses.
2. While both severity according to the TIMI scale and the need for RBC transfusion were equally distributed between access-site and non access-site related bleeding events, only the latter were associated
with a higher death or MI rate after one year of follow-up in both the univariate and multivariable-adjusted regression analysis.
The main results of the MULTISTRATEGY trial have been reported and discussed elsewhere13. The actual sub-analysis implementing the TIMI bleeding grading was pre-specified. The differential clinical impact of bleeding by different classifications among patients with ACS must be recognised14. Of interest, the overall incidence of both TIMI major and/or minor bleeding grading events within 30 days on the basis of clinical and laboratory findings was lower in the MULTISTRATEGY trial as compared to the Harmonising Outcomes with Revascularisation and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, and mirror the results of the Tirofiban arm in Ongoing Tirofiban in Myocardial Evaluation (On-TIME) 2 trial15-18. These findings may partly be explained by a less strict heparin protocol used in the HORIZONS-AMI trial, which had a target activated clotting time of 250 seconds but reached a median value in the GPI arm of 265 as compared to 232 seconds in our study. Moreover, patients with a recent (within six months), history of active bleeding were not eligible for participation in the MULTISTRATEGY trial12,13.
In our trial, nearly half of all TIMI major/minor bleeds within 30 days were access-site related, including retroperitoneal bleeds. However, they only independently contributed for about 1% of the deaths observed within 12 months. Our data are consistent with a recent observation by the STEEPLE trial investigators but contrast with the 4% mortality risk attributable to femoral access-related bleeding observed in a large contemporary PCI cohort19,20. While considering the large upper boundary of the 95% CI for the adjusted HR of 12-month mortality or MI by access-site related bleed (Figure 4), we acknowledge that we may have missed this signal by a lack of statistical power. Routine implementation of a transradial approach was previously demonstrated to dramatically reduce access-site bleedings, including TIMI major and minor bleedings21-23. However, whether and to which extent a transradial approach would independently translate into a mortality benefit in pPCI still merits further scrutiny. To this extent, the recently published RIVAL study, embedded within the CURRENT-OASIS 7 trial, already provided a strong signal towards a differential benefit of the transradial over the transfemoral vascular access strategy among patients undergoing primary, rescue, or urgent PCI for STEMI22. However, patients with STEMI comprised only one third of the RIVAL study population, and the broad confidence interval for the primary endpoint weakened the robustness of the data.
In contrast, in our study, non access- (versus access-) site related bleeding following pPCI contributed four to five times more to mortality. Early upper gastrointestinal (GI) bleed is a serious condition in ACS and is often associated with multiple-unit blood transfusion (90% of the patients with upper GI bleed in our trial had a transfusion, data not shown23). It was the most common source of non access-site related bleeding. Because patients at high bleeding risk were excluded from participation in MULTISTRATEGY, the true incidence of GI bleed in an unselected, more elderly, population may be even higher. Early concomitant use of proton blockers may prove effective in reducing bleeding events without any safety penalty towards an increase in ischaemic complications24. Several reasons could explain why bleeding leads to higher rates of cardiovascular events and deaths25. Non access-site bleeding may be more frequent in patients with high rates of co-morbidity, who therefore have intrinsically increased risks of adverse events.
Of interest, packed red blood cell transfusion at any or higher quantity was not causally related to mortality at 12 months in the MULTISTRATEGY trial. Again, this lack of relationship may reflect the insufficient power of a low signal-to-noise ratio considering the complex relationship between transfusion, bleeding and mortality. The direct link between transfusion and mortality was questioned in the APEX-AMI trial, which failed to demonstrate a dose–response effect of transfusion26.
Our findings on the predictors, risks and consequences of bleeding are in accordance with the literature27. Increasing age, gender, diabetes, renal dysfunction and IABP use have repeatedly been reported as independent predictors for significant bleeding in ACS or PCI. We also found that clinical signs of heart failure (KILLIP class) were associated with the occurrence of significant bleeding. Assessing the individual patient bleeding risk may inform decisions regarding bleeding avoidance strategies including the choice of the appropriate periprocedural antithrombotic regimen28,29.
Several limitations of the present study must be emphasized. We should acknowledge that MULTISTRATEGY is a relatively small, randomised controlled trial. The limited patient sample and the exclusion of patients at high risk of bleeding may limit the robustness of some of our findings. However, consistency of most of our results with previous work may substantiate the validity of our analysis. The logistic complexities of this trial imposed an open-label design with a potential for bias. We attempted to mitigate this potential for bias with the requirement that all ischaemic and bleeding events were adjudicated by independent committees unaware of the treatment assignments. In up to one quarter of patients, no overt bleeding source could be identified. These patients were classified as non access-site related bleeding. In all patients, there was a significant drop in haemoglobin; there was no confirmed retroperitoneal bleeding in any of them. However, whether asymptomatic decreases in haemoglobin and/or haematocrit that do not require transfusion impact prognosis is questionable14.
In conclusion, the results of our study indicate that TIMI major and minor grade bleedings that occur within 30 days following pPCI identify patients who are at risk for intermediate-term death or MI. However, access-site related bleeding, while substantially contributing to the total bleeding events, did not contribute to 12-month mortality and MI to the same extent as non access-site related bleeding in this analysis. Understanding the mechanisms by which bleeding is causally related to adverse clinical events is crucial in developing strategies to reduce the risks of adverse outcomes in ACS.
Conflict of interest statement
The authors have no conflicts of interest to declare.

