
Abstract
Aims: We sought to evaluate bleeding complications and periprocedural outcomes of the radial approach (RA) as compared to the femoral approach (FA) during percutaneous coronary intervention (PCI) in “real-world” patients with ST-segment elevation myocardial infarction (STEMI).
Methods and results: The study group consisted of 22,812 consecutive patients with STEMI treated with PCI and stent implantation between January 2014 and June 2015 in 151 tertiary invasive cardiology centres in Poland (the ORPKI Polish National Registry). Patients treated using the RA and FA were compared using a propensity score analysis to avoid possible selection bias. The analysis was carried out in an “as-treated” manner. The FA was used in 9,334 (40.9%) and the RA in 13,478 (59.1%) patients. After propensity score matching, a higher total amount of contrast (191.8±8.0 vs. 174.8±68.8 ml; p=0.001) and lower radiation doses (1,279.5±1,346.3 vs. 1,182.6±887 mGy; p=0.02) were reported in FA. More access-site-related bleeding complications after both angiography (0.17% vs. 0.02%; p=0.004) and PCI (0.23% vs. 0.09%; p=0.049) were reported in the FA group. Periprocedural death (1.94% vs. 0.93%; p=0.001) was more common after PCI performed with the FA.
Conclusions: The radial approach was associated with a lower incidence of periprocedural death in STEMI patients as well as a significant reduction of bleeding complications at the access site.
Abbreviations
ACS: acute coronary syndrome
FA: femoral approach
PCI: percutaneous coronary intervention
RA: radial approach
STEMI: ST-segment elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
Introduction
The utilisation of the radial approach (RA) for percutaneous coronary intervention (PCI) has gradually increased1. Numerous studies have shown that the radial artery is a safe alternative to the femoral artery as a vascular access site for coronary angiography and PCI in acute coronary syndrome (ACS)2-9. Although technically more demanding, RA has been demonstrated to be as feasible as the femoral approach (FA) in the setting of ST-segment elevation myocardial infarction (STEMI)2-4. Importantly, in STEMI a greater use of potent adjunctive antithrombotic and antiplatelet agents is required. On the other hand, the use of these agents potentially leads to higher bleeding rates and vascular complications. These complications were shown to be associated with adverse long-term outcome after PCI. Thus, the most benefit from RA is expected in patients with STEMI. Current European Society of Cardiology (ESC) guidelines recommend the radial over the femoral route for primary PCI in STEMI for experienced operators10. Despite the growing body of evidence in favour of the RA, there are still limited data on the clinical outcomes in an all-comers population of patients with STEMI. More data are needed from an unselected cohort of patients in the era of universal use of the RA by operators with different levels of expertise, not only from high-volume centres. Thus, we sought to evaluate bleeding complications and periprocedural outcomes of RA as compared to FA during PCI with stent implantation in “real-world” patients with STEMI enrolled in the Polish National PCI Registry (ORPKI).
Methods
This registry was a prospective, non-randomised, observational study which consisted of 22,812 consecutive patients with STEMI treated with PCI and stent implantation between January 2014 and June 2015 in 151 tertiary invasive cardiology centres in Poland. All collected data were stored in the electronic database of the ORPKI Registry operated by the Jagiellonian University Medical College in Krakow, Poland, and endorsed by the Polish Association of Cardiovascular Interventions of the Polish Cardiac Society6,11. Data in the ORPKI registry have been gathered via electronic case report forms (CRF) in the majority of interventional cardiology centres in Poland since 20046,11. No personal data are collected in the registry.
PCI was performed using either the RA or the FA per the operator’s discretion. Vascular access site (radial or femoral) was defined as the site of successful vascular entry. Failed attempts and the crossover rates were not captured. Target lesion selection and treatment technique were also left to the operator’s discretion. Complexity and lesion type were not collected. Standard balloon catheters and stents were used. All procedures were carried out according to local standards of PCI and ESC guidelines wherever applicable. All procedures were carried out by operators with different expertise levels in RA. All complications which occurred during the procedure were documented prospectively. Periprocedural mortality was defined as death of any cause during PCI and until transfer from catheterisation laboratory to cardiology department or intensive care unit. Bleeding complications were defined homogeneously in all centres as any overt, actionable sign of haemorrhage (e.g., more bleeding than would be expected for a clinical circumstance, including bleeding found by imaging alone) that did not fit the criteria for type 3, 4, or 5 but did meet at least one of the following criteria: (1) requiring non-surgical, medical intervention by a healthcare professional, (2) leading to hospitalisation or increased level of care, or (3) prompting evaluation12. Adverse events were diagnosed at the operator’s discretion according to the definitions in current ESC guidelines. No further evaluation or follow-up was performed after hospital discharge. All patients provided informed consent for the procedure. The study complied with the ethical principles for clinical research based on the Declaration of Helsinki with later amendments. No funding was used to support this registry.
STATISTICAL ANALYSIS
To avoid the potential influence of the non-randomised design, a propensity score was calculated using a multivariate logistic regression model with the route of intervention (radial versus femoral) considered as the dependent variable. All baseline characteristics (gender, age, weight, diabetes mellitus, previous stroke, previous myocardial infarction, previous PCI, previous coronary artery bypass grafting, smoking status, chronic kidney disease, pre-hospital treatment: acetylsalicylic acid, P2Y12 inhibitors, unfractionated heparin, low-molecular-weight heparin; baseline clinical data: thrombolysis, sudden cardiac arrest, hypothermia, Killip-Kimball class, Thrombolysis In Myocardial Infarction [TIMI] scale before PCI) were set as covariates. A cut-off of calliper of 0.01 was used to obtain satisfactory balancing, that is if standardised differences for all confounders were estimated as below 10%. Patients were matched into a 1:1 design. Unpaired patients were discarded from analysis.
Standard descriptive statistics were used in the analysis. Quantitative variables were described using mean and standard deviation. Categorical variables were presented as counts and percentages. The Mann-Whitney U test (for non-normally distributed data) or the Student’s t-test (for normally distributed data) for continuous variables and Fisher’s exact test or the Pearson’s chi-squared test for categorical (nominal and dichotomous) variables were used to compare groups before matching. The normality of the data was assessed with the KSL test. Matched pairs of subjects were compared with the Wilcoxon signed-rank test (for non-normally distributed data difference) or the paired t-test (for normally distributed data difference) for continuous variables and the McNemar-Bowker’s test for categorical (nominal and dichotomous) variables. The level of statistical significance was set at p<0.05. The analysis was carried out in the “as-treated” manner. In addition, analysis with whole radial and femoral populations using standard multivariate adjustment was calculated. Forward selection in logistic regression analysis with a probability value for covariates to enter the model was set at the 0.05 level. Results were presented as odds ratios (OR) with 95% confidence intervals (CI). All calculations were performed with JMP 9.0.0 software (SAS Institute Inc., Cary, NC, USA).
Results
FA and RA were used in 9,334 (40.9%) and 13,478 (59.1%) patients, respectively. Complete baseline clinical and demographic characteristics of the included patients are presented in Table 1. After the propensity score match no significant differences in baseline characteristic data were found between the analysed groups. A total of 6,542 matched pairs with STEMI treated with PCI via RA or FA were included in the analysis. A flow chart of the patients is presented in Figure 1.
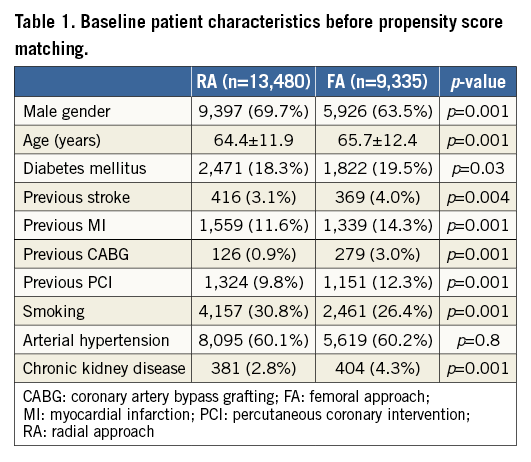
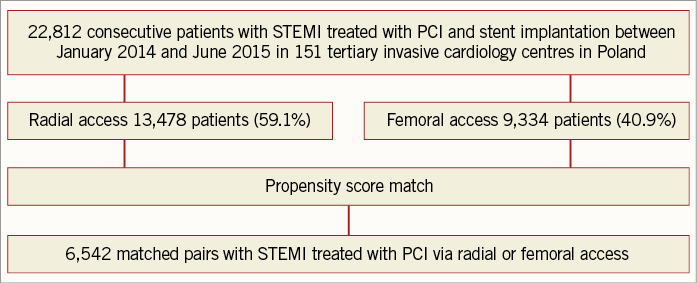
Figure 1. Flow chart of included patients.
All the following data were calculated for matched pairs. No differences in the ratio of direct transport to the catheterisation laboratory and out-of-hospital sudden cardiac arrest were observed between the groups (RA vs. FA, respectively: 23.7% vs. 24.8%; p=0.1; 4.9% vs. 5.1%, for both p=0.7). Data on pre-hospital treatment are presented in Table 2. A similar prevalence of Killip-Kimball class IV presentation at admission to the catheterisation laboratory was found in both groups (FA vs. RA, respectively: 3.1% vs. 2.9%; p=0.8). No differences in the angiographic indications for PCI and target lesion location were observed (Table 3). Details of PCI and both antiplatelet and antithrombotic therapy during the procedures are presented in Table 4. No difference in blood flow in the target vessel measured with the TIMI scale was observed before PCI (p=0.7). On the other hand, TIMI grade 3 flow was more common after PCI with the RA (91.5% vs. 89.7%; p=0.002) (Table 3). The RA was associated with a lower incidence of periprocedural death in STEMI patients as well as a significant reduction of bleeding complications at the access site after both angiography and PCI. The periprocedural results of PCI are presented in Table 5.
In multivariate analysis of all 22,812 consecutive patients, RA was associated with lower risk of periprocedural death (OR 0.503, 95% CI: 0.3769-0.6713; p=0.0001) and bleeding at the puncture site after both angiography and PCI (respectively, OR 0.1021, 95% CI: 0.0221-0.4713; p=0.0004; OR 0.31, 95% CI: 0.134-0.717; p=0.005). Furthermore, subgroup analysis with all 22,812 patients was performed for periprocedural death (Figure 2).
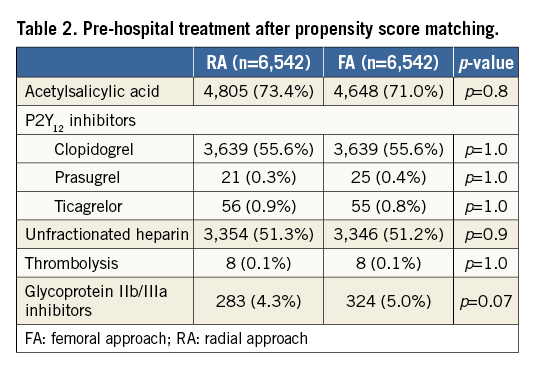
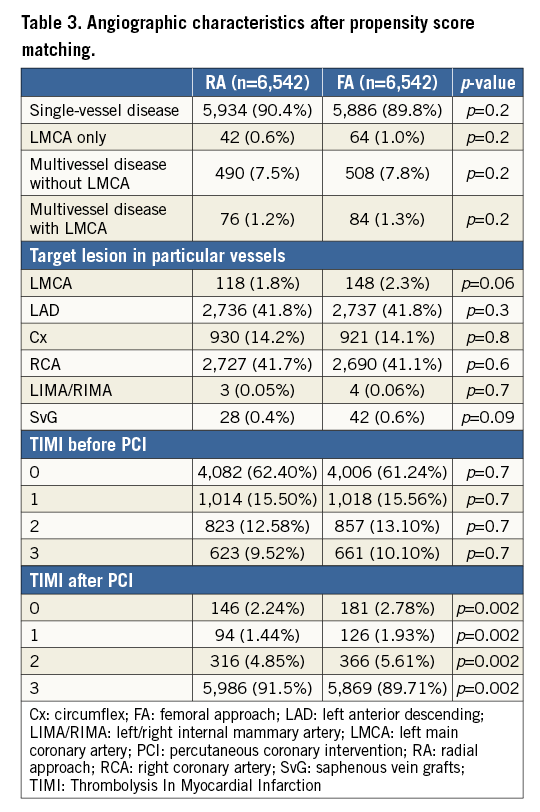
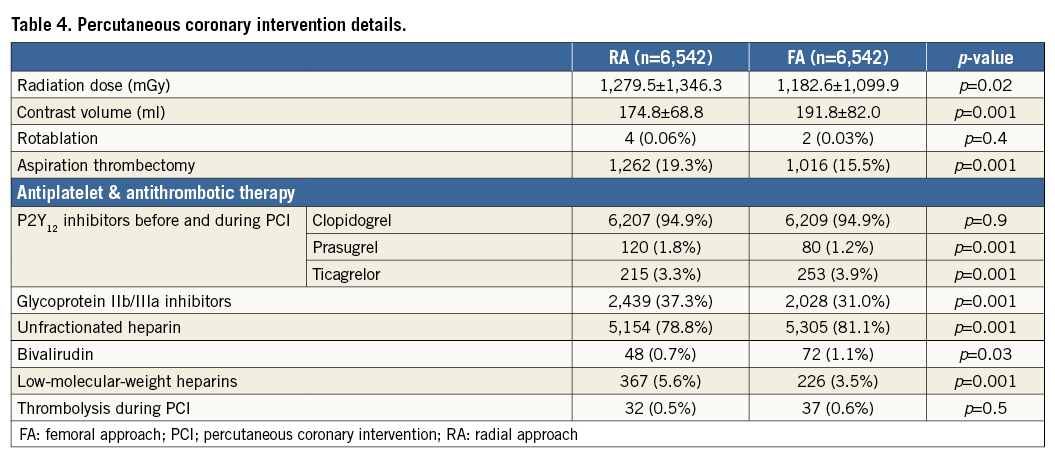
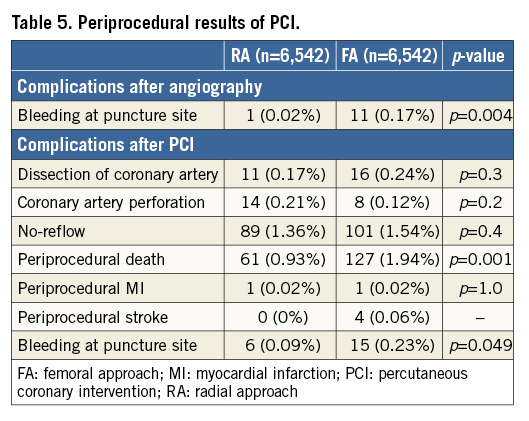
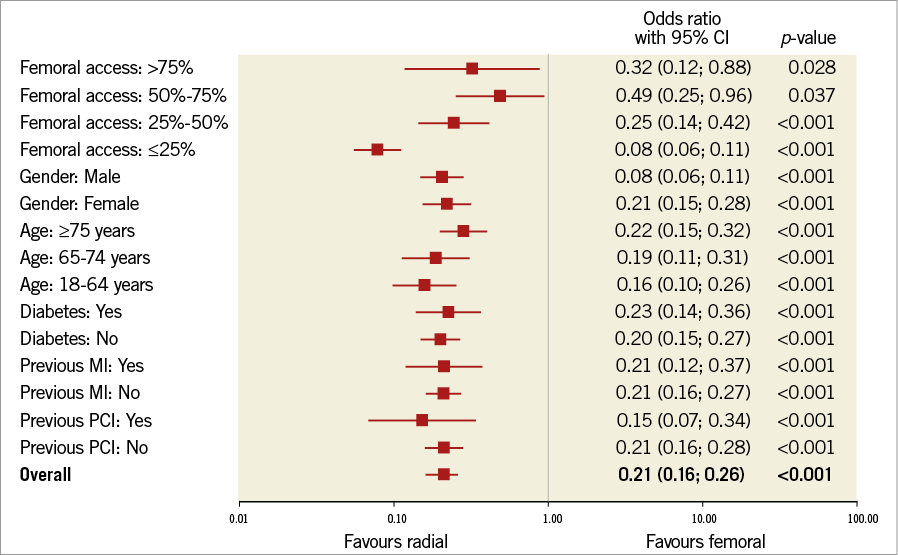
Figure 2. Forest plot for periprocedural death with subgroup analysis and for all included patients.
Discussion
The results of a large cohort of patients from the ORPKI Registry demonstrated lower rates of periprocedural bleeding complications and mortality for the RA during PCI with stent implantation in STEMI patients as compared to the FA. These findings support current recommendations for RA in STEMI8.
Similar benefits in outcome with RA in STEMI have been reported in previous randomised controlled trials and their meta-analyses2-4,10,13-15. In two large trials, RA reduced the mortality and bleeding complication rates in patients with STEMI3,4,10. However, these studies had several limitations. In the STEMI subgroup of the RIVAL trial, only 74% of patients underwent primary PCI3. Most of the deaths were noted in patients who did not have a major bleeding or an access-site complication3. There was no stratification according to clinical presentation, and the STEMI population originated from a post hoc analysis of a limited group (28%) of patients involved in the trial3. Thus, the results of subgroup analysis should be interpreted with caution. In the RIFLE-STEACS trial, patients with cardiogenic shock were included and the majority of deaths were from pump failure and not related to access-site complications or bleeding4. In both studies, investigators included patients after thrombolysis and with symptom duration up to 24 hours3,4. In another randomised trial, a significant reduction in bleeding and access-site complications with RA was presented; however, there was no difference between the two approaches in terms of mortality2. Data regarding the reduction in bleeding and mortality rates with RA in the current analysis are consistent with a recent large registry16.
In RIVAL, there was an interaction between the benefit of RA and operator experience, suggesting that the superiority of RA over FA depends upon radial expertise3,13. In our “real-world” registry, reduced periprocedural mortality and bleeding rates were demonstrated despite a wide range of experience in RA. However, a lower risk of periprocedural death was reported in centres with predominant RA utilisation. The data presented are consistent with results from the MATRIX trial8. On the other hand, a recent study reported a higher prevalence of stroke during PCI in acute myocardial infarction performed by the operators with less experience with the RA17. In the logistic regression analysis, the percent of PCIs using the RA per operator (OR 0.981 per 1% increase, 95% CI: 0.967-0.997; p=0.02) was identified as an independent predictor of periprocedural stroke17. More interestingly, contrary to a recent report from the ACCOAST trial, no impact of the RA per se on the risk of stroke was confirmed17-19. In our study, a low incidence of periprocedural stroke with no differences between groups was observed.
Percutaneous procedures with the RA have also been shown to reduce the incidence of acute bleeding complications, especially in ACS7. Major bleeding events have been demonstrated to impact negatively on prognosis20-22, while minor bleeding may force the discontinuation of dual antiplatelet therapy, with a direct increased risk of stent thrombosis and adverse events22. In our registry, pre-hospital anticoagulant and antithrombotic therapies were similar in both groups. Furthermore, glycoprotein IIb/IIIa inhibitors were significantly more frequently administered during procedures with the RA in comparison with the FA group, which also supports the idea of a beneficial influence of RA in decreasing the risk of bleeding complications. Bivalirudin was more often used during PCI with FA. However, recent studies have posed questions on the advantage of bivalirudin compared to heparin for reducing the risk of major bleeding23,24. No superiority over unfractionated heparin was demonstrated recently in patients with ACS24. Most of the benefit of bivalirudin has been demonstrated in patients undergoing PCI with the FA25 and compared with heparin and glycoprotein IIb/IIIa inhibitors26.
According to the data presented in our analysis, the benefits in the mortality rate cannot be explained by differences in baseline characteristics, pre-hospital treatment, periprocedural rates of myocardial infarction, stroke or coronary artery perforation, which were similar in both groups after propensity score matching. In previous studies, favourable outcome in RA might be partly related to the reduction of bleeding and access-site complications27. Blood transfusions and the interruption of antiplatelet or antithrombotic therapy are associated with a higher mortality in patients after PCI with STEMI27,28. Hence, RA which decreases the risk of access-site-related bleeding can directly influence outcomes after primary PCI in the setting of STEMI. However, a direct cause-effect association between periprocedural death and bleeding complications cannot be confirmed in our analysis. Reduced periprocedural mortality in RA should be considered as an independent outcome, not as a result of less frequent bleeding at the access site.
The reduction in mortality might be partially explained by limitations related to the available data. Propensity score might not have sufficiently corrected the results for hypothetical unmeasured confounders such as left ventricular ejection fraction, which might have compelled the operators to select the FA for patients at higher risk.
Despite the postulated advantages, many operators consider RA as more technically challenging. Potential radial artery spasm with high anatomic variability and subclavian tortuosity are the main limitations that discourage operators. Radial artery access in PCI may lead to longer fluoroscopy times and higher radiation doses, as well as a potentially longer time to successful reperfusion. A recent study postulated no difference in radiation exposure in high-volume centres and operators, and this can be overcome with increased training and expertise29. Previous studies have reported significantly higher radiation doses in patients undergoing percutaneous procedures with the RA29,30. Similar results were observed in our registry. Various factors might influence radiation exposure, including operator experience, patient and lesion characteristics. Furthermore, in our registry the load of contrast was significantly lower in RA patients. Similar data were reported in a recent registry and randomised trial2,27. This might reflect a difference in operator experience between the groups. It could also be related to differences in the complexity of procedures performed with RA and FA. However, these data were not captured in our study. According to the data presented, vascular access might be an important modifiable risk factor in STEMI patients treated with PCI.
The radial approach is evolving into the standard access site for percutaneous procedures. The future holds promise for RA in PCI in terms of clinical outcome, patient comfort in rapid mobilisation, earlier discharge and improved quality of life15,18. On the other hand, the common and still growing use of the RA might lead to a problem with decreasing experience in PCI using the femoral artery. The safety and efficacy of this route could be a potential problem in the near future for interventionists practising mostly with the radial artery.
Limitations
The main limitation of this study is the non-randomised design. The possibility of unmeasured confounders influencing the outcome rates cannot be excluded. We tried to overcome this limitation by utilising a propensity matching. We could not exclude the influence of differences in the experience of the operators. RA may be preferentially selected by more skilled operators. Of note, the decision regarding the route of intervention was at the operator’s discretion.
Another major limitation is related to the lack of some clinical data. Importantly, we could not estimate the access crossover rates. Access failures and femoral/radial re-access rates could not be evaluated. Furthermore, in-hospital outcomes and data beyond hospital discharge were not collected. Thus, the incidence of radial artery occlusions after the procedures was not captured.
The size of vascular sheaths used during PCI was not reported. There are no data regarding utilisation of closure devices in the femoral group. Since there is a lack of some clinical data, propensity score might not be sufficient to eliminate the influence of hypothetical unmeasured confounders. We have reported the results from the experience of 151 centres and, despite all of the limitations, our data reflect the outcome of a “real-world” population and a “real-world” experience which is different from that selected in randomised controlled trials. Thus the results can be extrapolated to the general population.
Conclusions
The radial approach was associated with improved outcome in comparison with the FA in patients with STEMI undergoing PCI and stent implantation. A significant reduction of access-site-related bleeding complications after both angiography and PCI was observed in the RA group. A lower rate of periprocedural mortality was reported with the RA.
| Impact on daily practice We demonstrated a lower rate of periprocedural bleeding complications and mortality for the RA during PCI with stent implantation in STEMI patients as compared to the FA. Vascular access might be an important modifiable risk factor in STEMI patients treated with PCI. The common and still growing use of the RA could result in a problem with decreasing experience in PCI with FA utilisation. The safety and efficacy of this route could be a potential problem in the near future for interventionists practising mostly with radial artery access. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

