Abstract
Aims: We sought to determine whether a transradial (TR) approach compared with a transfemoral (TF) approach was associated with improved clinical outcomes in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) in a post hoc analysis of the HORIZONS-AMI trial. There is a paucity of data comparing the TR approach with the TF approach in patients with STEMI treated with primary PCI and contemporary anticoagulant regimens.
Methods and results: In HORIZONS-AMI, primary PCI for STEMI was performed in 3,340 patients, either by the TR (n=200) or TF approach (n=3,134). Endpoints included the 30-day and one-year rates of major adverse cardiovascular events (MACE: death, reinfarction, stroke or target vessel revascularisation), non CABG-related major bleeding, and net adverse clinical events (NACE: MACE or major bleeding). TR compared to TF access was associated with significantly lower 30-day rates of composite death or reinfarction (1.0% vs. 4.3%, OR 0.23, 95% CI [0.06,0.94], p=0.02), non CABG-related major bleeding (3.5% vs. 7.6%, OR 0.45, 95% CI [0.21,0.95], p=0.03), MACE (2.0% vs. 5.6%, OR 0.35, 95% CI [0.13,0.95], p=0.02), and NACE (5.0% vs. 11.6%,OR 0.42, 95% CI [0.22,0.78], p<0.01). At one year, the TR group still had significantly reduced rates of death or reinfarction (4.0% vs. 7.8%, OR 0.51, 95% CI [0.25,1.02], p=0.05), non CABG-related major bleeding (3.5% vs. 8.1%, OR 0.42, 95% CI [0.20,0.89], p=0.02), MACE (6.0% vs. 12.4%, OR 0.47, 95% CI [0.26,0.83], p<0.01) and NACE (8.5% vs. 17.8%, OR 0.45, 95% CI [0.28,0.74], p<0.001). By multivariable analysis, TR access was an independent predictor of freedom from MACE and NACE at 30 days and one year.
Conclusions: In patients with STEMI undergoing primary PCI with contemporary anticoagulation regimens in the HORIZONS-AMI trial, a TR compared with a TF approach was associated with reduced major bleeding and improved event-free survival.
Abbreviations and acronyms
ACEI: angiotensin-converting enzyme inhibitor
BMI: body mass index
BMS: bare metal stent
CABG: coronary artery bypass graft
CAD: coronary artery disease
DES: drug-eluting stent
GP IIb/IIIa: glycoprotein IIb/IIIa
GUSTO: global utilisation of streptokinase and tissue plasminogen activator for occluded coronary arteries
HORIZONS-AMI: Harmonizing Outcomes with RevascularIZatiON and Stents in Acute Myocardial Infarction
MACE: major adverse cardiovascular events
NACE: net adverse clinical events
PCI: percutaneous coronary intervention
PES: paclitaxel-eluting stent
RIVAL: Radial Versus Femoral Access for Coronary Angiography and Intervention in Patients with Acute Coronary Syndromes
STEMI: ST-segment elevation myocardial infarction
SVG: saphenous vein graft
TF: transfemoral
TIMI: Thrombolysis In Myocardial Infarction
TR: transradial
UFH: unfractionated heparin
Introduction
Primary percutaneous coronary intervention (PCI) and antithrombotic therapies have significantly improved the prognosis of patients with ST-segment elevation myocardial infarction (STEMI1,2) Nonetheless, post-PCI bleeding complications occur frequently and are associated with a poor prognosis3-7. Growing evidence has demonstrated a strong correlation between bleeding, ischaemic events and mortality in patients undergoing PCI, and recent studies emphasise reduction of bleeding as a major therapeutic goal for patients receiving antithrombotic therapy and those undergoing PCI8-14. Compared with transfemoral (TF) arterial access, vascular access via the transradial (TR) artery has been associated with a significant decrease in major and minor bleeding, early ambulation, less patient discomfort , as well as reduced intra-hospital cost15-17. Benefits associated with TR access have been found across the spectrum of patients undergoing PCI, including those with STEMI18-20. Nonetheless, the TR approach is infrequently used, accounting for only 1% of the total PCI procedures being performed in the United States21, although its adoption has recently been increasing. There is also a relative paucity of data comparing the TR and TF approaches in STEMI22-30; with most studies having been limited by their retrospective nature23,30, small sample sizes23-27,29 and/or short-term follow-up22-24,26-29. Recently, the multicentre randomised RIVAL trial (radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes) has been published, and while overall negative, suggested that TR compared to TF access may be superior in a STEMI population20. Of note, however, less than 3% of the patients received bivalirudin in the RIVAL trial. We therefore evaluated the impact of the TR approach on the 30-day and one-year clinical outcomes in patients with STEMI enrolled in the Harmonizing Outcomes with RevascularIZatiON and Stents (HORIZONS-AMI) trial, a large-scale, contemporary international study of patients undergoing primary PCI with contemporary antithrombotic regimens.
Methods
Patient population and study protocol
The HORIZONS-AMI trial design and patient eligibility criteria have been previously described in detail2. In brief, HORIZONS-AMI was a prospective, open-label, randomised, multicentre trial enrolling 3,602 patients at 123 international centres with STEMI who presented within 12 hours after the onset of symptoms for primary PCI. Patients were randomised in a 1:1 ratio in the emergency room to administration of anticoagulation with unfractionated heparin (UFH) plus a GP IIb/IIIa inhibitor (active control group) or bivalirudin monotherapy. Following angiography, 3,006 PCI patients with lesions eligible for stenting underwent a second randomisation in a 3:1 ratio to either TAXUS® Express2™ paclitaxel-eluting stents (PES) or otherwise identical Express2™ bare metal stents (BMS) (both: Boston Scientific, Natick, MA, USA). The present analysis is restricted to patients who received at least one stent (either a DES or BMS) during the index procedure, whether or not in the stent randomisation arm. Clinical follow-up was pre-specified at 30 days, six months, one year, and yearly thereafter for five years.
Access site: radial vs. femoral
The choice of access route was left to the discretion of the investigator. All analyses are reported according to the actual access site used to perform the PCI. A clinical events committee blinded to treatment assignment adjudicated all endpoint events using original source documents. The study was approved by the institutional review board or ethics committee at each participating centre, and all patients signed informed consent.
Endpoints and definitions
The endpoint definitions in the HORIZONS-AMI trial have been previously reported in detail2. Two primary endpoints were pre-specified: major bleeding not related to coronary artery bypass grafting (CABG), and net adverse clinical events (NACE), defined as the combination of major bleeding or a composite of major adverse cardiovascular events (MACE), including death, reinfarction, target vessel revascularisation for ischaemia, or stroke. Major bleeding was defined as intracranial or intraocular haemorrhage, haematoma ≥5 cm in diameter, access site haemorrhage requiring intervention, reoperation for bleeding, clinically overt bleeding with a decrease in haemoglobin by ≥3 g/dl, reduction in haemoglobin concentration of ≥4 g/dl without an overt source of bleeding, or the need for any blood product transfusion31. Bleeding events were also graded using the Thrombolysis In Myocardial Infarction (TIMI32) and global utilisation of streptokinase and tissue plasminogen activator for occluded coronary arteries (GUSTO33) classifications. The present sub-analysis reports these endpoint measures and their components at 30-day and one-year follow-up, comparing primary PCI via TR access vs. TF access. Quantitative and qualitative coronary analysis was performed by an independent angiographic core laboratory (The Cardiovascular Research Foundation, New York, NY, USA) for baseline and final lesion and flow characteristics34.
Statistical analysis
Continuous variables were expressed as median and interquartile range and were compared using the Wilcoxon rank-sum test. Categorical variables were compared with the chi-square test or Fisher’s exact test. Kaplan Meier methods were used to estimate event rates at follow-up and to plot time-to-event curves; comparisons were made using the log-rank test. A Cox proportional hazards regression was performed to identify the independent predictors of 30-day and one-year adverse events (α=0.05). Interactions were tested by including the cross product of the two variables (an interaction term) in the Cox model. The multivariable models were built by stepwise variable selection with entry and exit criteria set at the p<0.1 level. To avoid over-fitting the models, the following potential covariates, which are known to affect ischaemic and/or bleeding outcomes in STEMI were included: age, sex, diabetes, Killip class, baseline haemoglobin, platelet and white blood cell counts, creatinine clearance, previous CABG, thienopyridine loading dose (clopidogrel 600 mg vs. other), use of heparin before randomisation, randomisation to bivalirudin vs. UFH + GP IIb/IIIa inhibitors, symptom onset to first balloon time, LAD infarct artery, and access site (TR vs. TF).
Results
A total of 3,602 patients were enrolled at 123 centres in 11 countries, 3,344 of whom underwent primary PCI, in whom the access site was identified as either TR (n=200; 6.0%) or TF (n=3,134). A total of 10 patients who underwent PCI via brachial access were excluded. Only 17 centres (13.8%) treated one or more enrolled patients via TR access. Figure 1 shows the proportion of cases completed by TR access among centres that enrolled at least one TR patient.
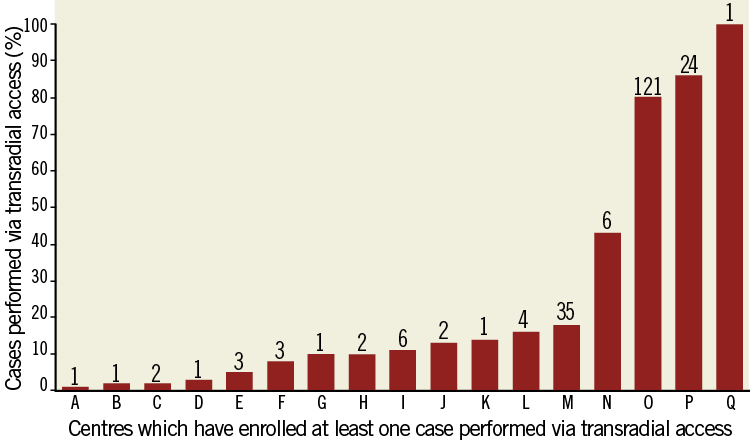
Figure 1. Proportion of cases done by TR approach among the 17 centres that have enrolled at least one case performed by TR access in the HORIZONS trial.
Study population
Clinical and angiographic characteristics of the two groups are shown in Tables 1-3. Compared to patients in the TR group, those in the TF group more frequently had hyperlipidaemia, previous CABG, and were more likely to have been treated with aspirin, a thienopyridine, and calcium-channel within five days prior to enrolment. Patients in the TF group received a 600 mg clopidogrel loading dose more frequently than did the TR group (66.2% vs. 30.8%; p<0.0001). Patients in the TF group also more frequently received bail-out GP IIb/IIIa inhibitors (13.7% vs. 2.9%; p<0.0001). There were no significant differences in lesion characteristics between the two groups with the exception of a slight excess of bifurcation lesions in the TR group and thrombotic lesions in the TF group.
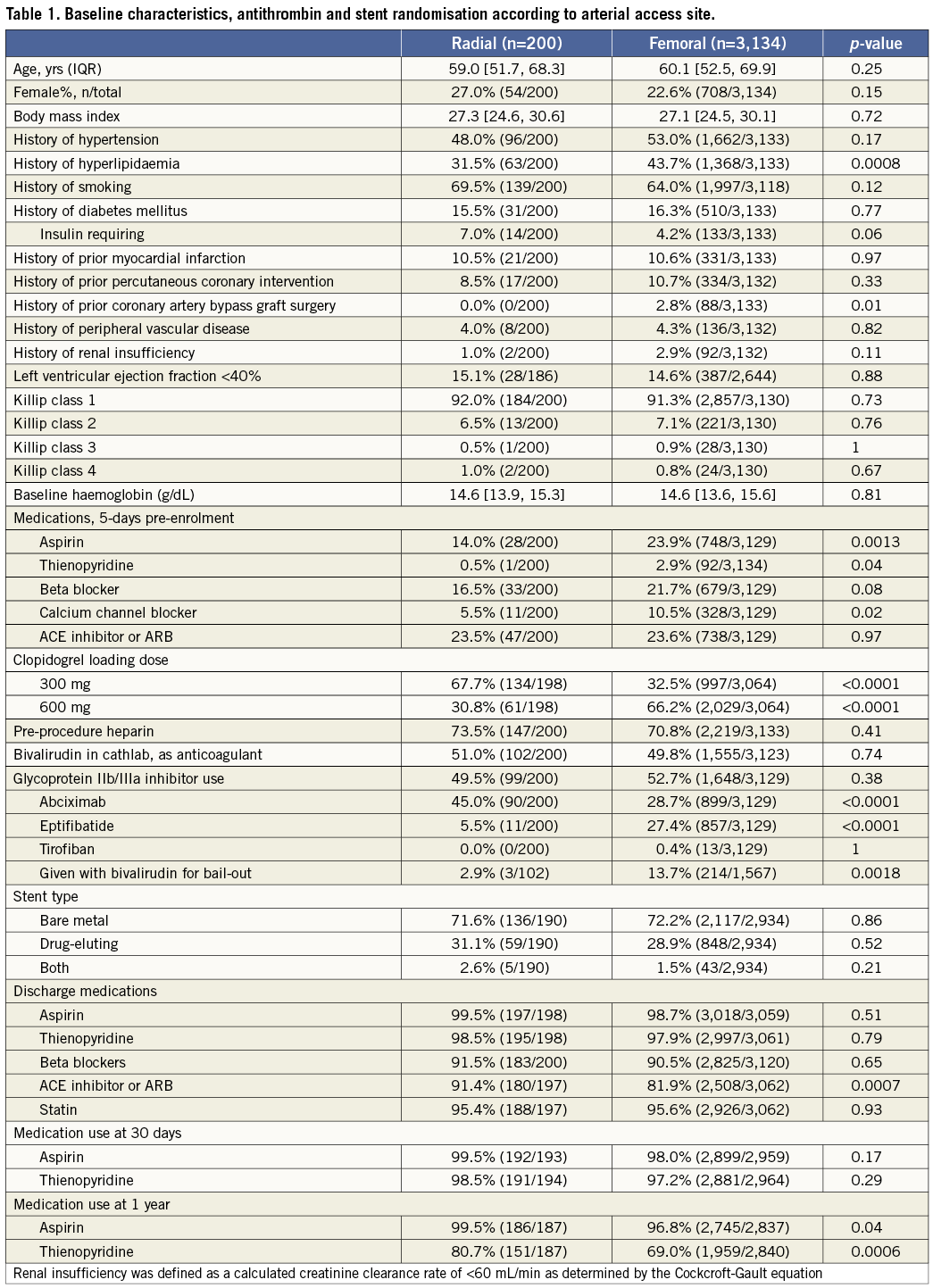
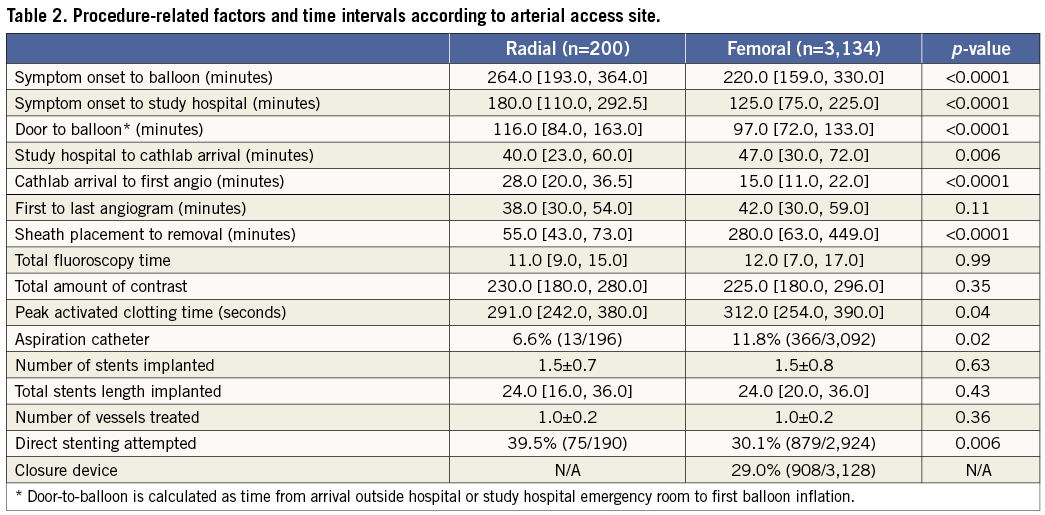
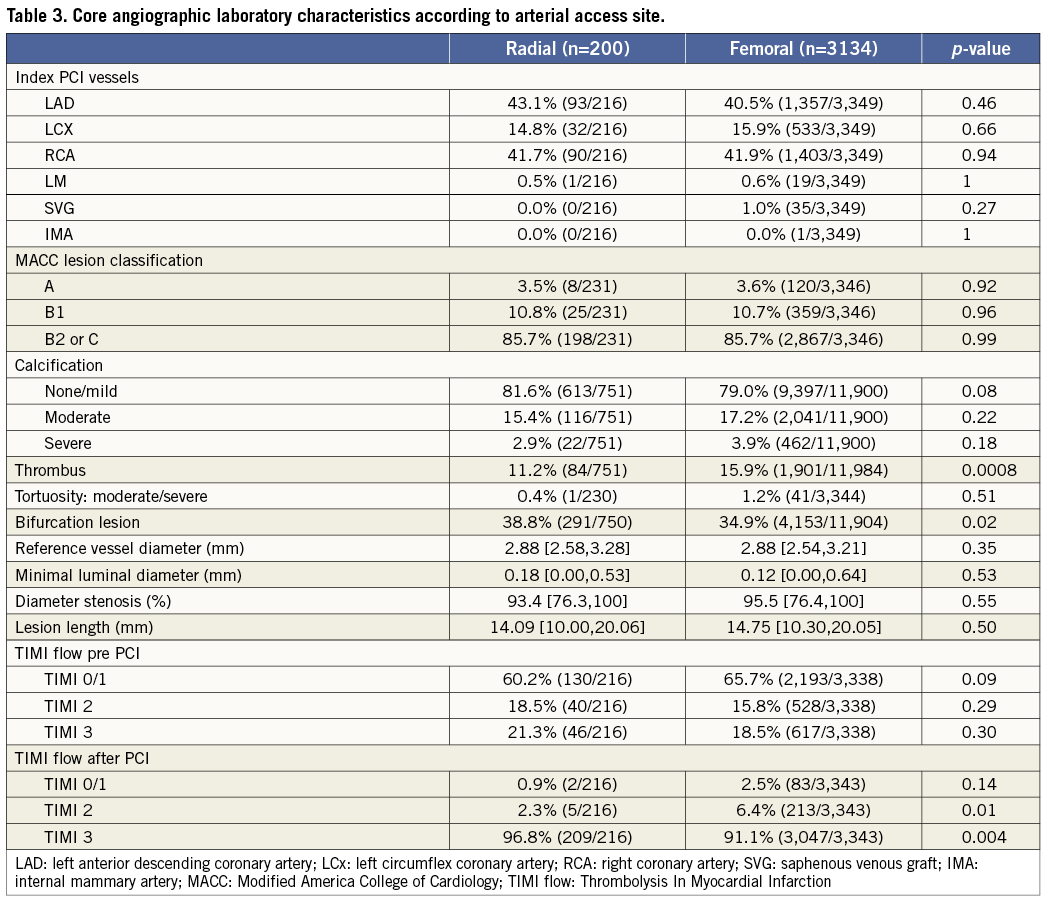
Door-to-balloon times were substantially longer in the TR group compared with the TF group (116 min vs. 97 min; p<0.0001), mainly due to a longer time from cathlab arrival to first angiogram (28 min vs. 15 min; p<0.0001). Total ischaemic time in the TR group was also longer, due both to a longer time from symptom onset to ER arrival, as well as from ER arrival to balloon inflation. However, total procedural time (from first angiogram to last angiogram), fluoroscopy, and amount of contrast used were similar in both groups. Post PCI TIMI-3 flow was present in a significantly higher proportion of patients in the TR group. Closure devices were used in 29% of the TF cases, including Angio-Seal™ (St. Jude Medical, St. Paul, MN, USA) in 58%, StarClose™ (Abbott Vascular, Redwood City, CA, USA) in 32% and Perclose™ (Abbott Vasuclar) in 10%.
Aspirin and thienopyridine treatment was similar in both groups at 30-day follow-up. However, patients in the TR group were more likely to have received aspirin and a thienopyridine at one-year follow-up.
Clinical outcomes
As shown in Table 4, patients in the TR compared with the TF group had significant reductions in the 30-day rates of non CABG-related major bleeding, TIMI and GUSTO bleeding, reinfarction, MACE and NACE. These findings persisted at one-year follow-up (Table 5 and Figure 2). Stent thrombosis rates were comparable between the two groups. By multivariable analysis, TR access was an independent predictor of freedom from NACE, MACE, and composite death or reinfarction at 30 days, with a non-statistically significant trend present for reduced major bleeding. At one-year, TR access was an independent predictor of freedom from NACE, MACE, and non-CABG major bleeding, with a non-statistically significant trend present for reduced composite death or reinfarction (Table 6). The lowest rates of adverse events occurred in patients randomised to bivalirudin who underwent PCI by TR access, whereas the highest event rates were observed in patients randomised to heparin plus GP IIb/IIIa inhibitors who underwent PCI by TF access (Figure 3).
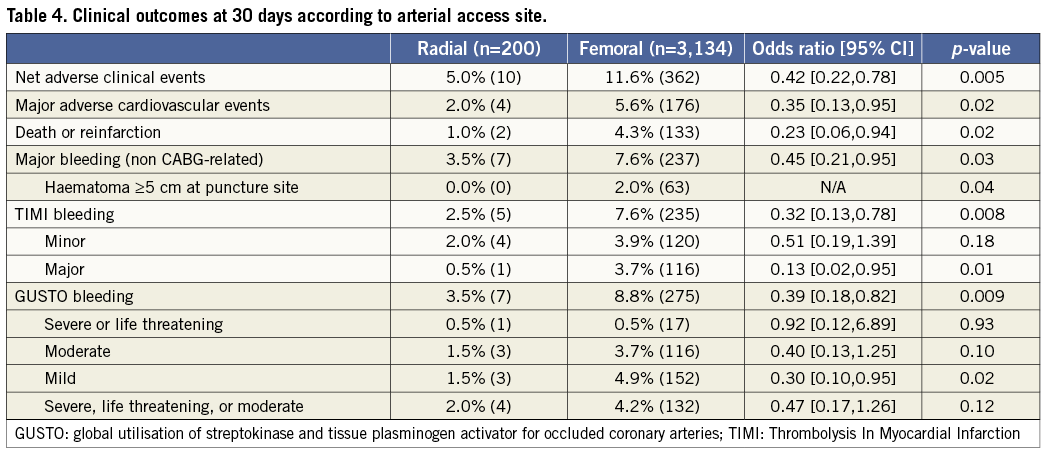
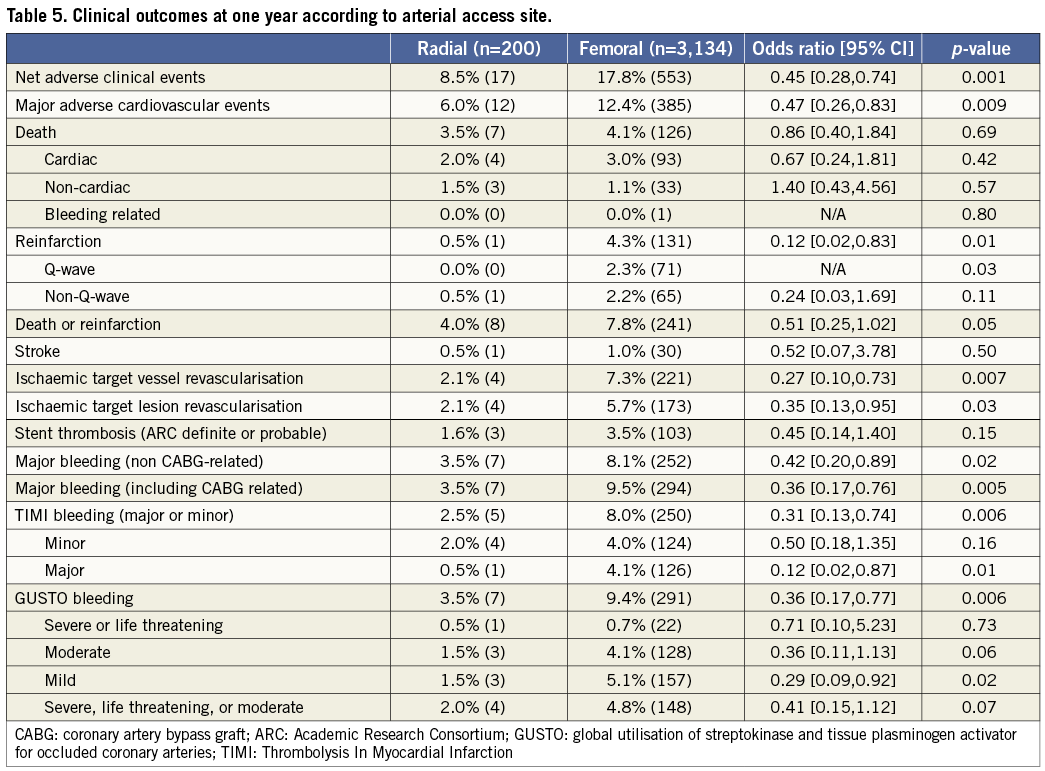
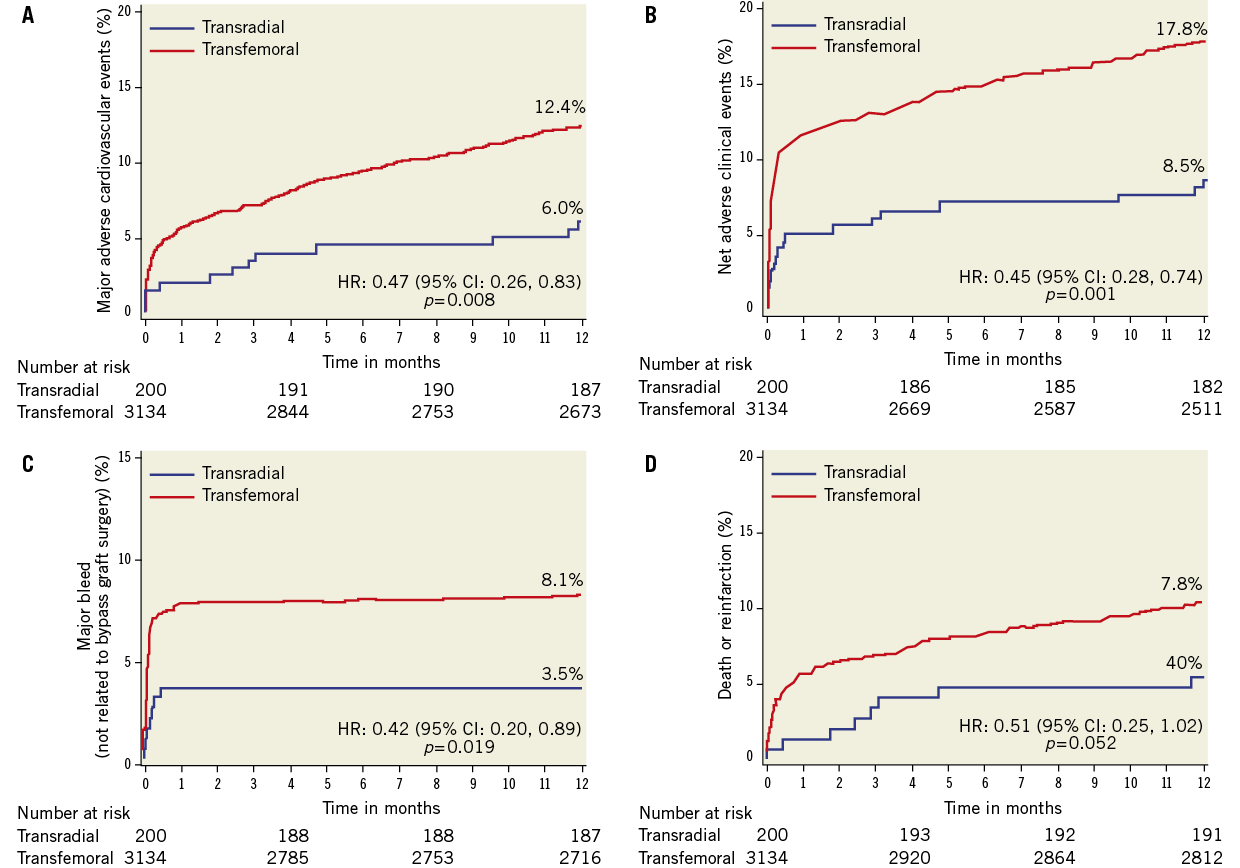
Figure 2. Time-to-event curves for one-year cumulative: A) NACE; B) MACE; C) non CABG-related major bleeding; and D) death or reinfarction in patients undergoing primary PCI with transradial vs. transfemoral access.
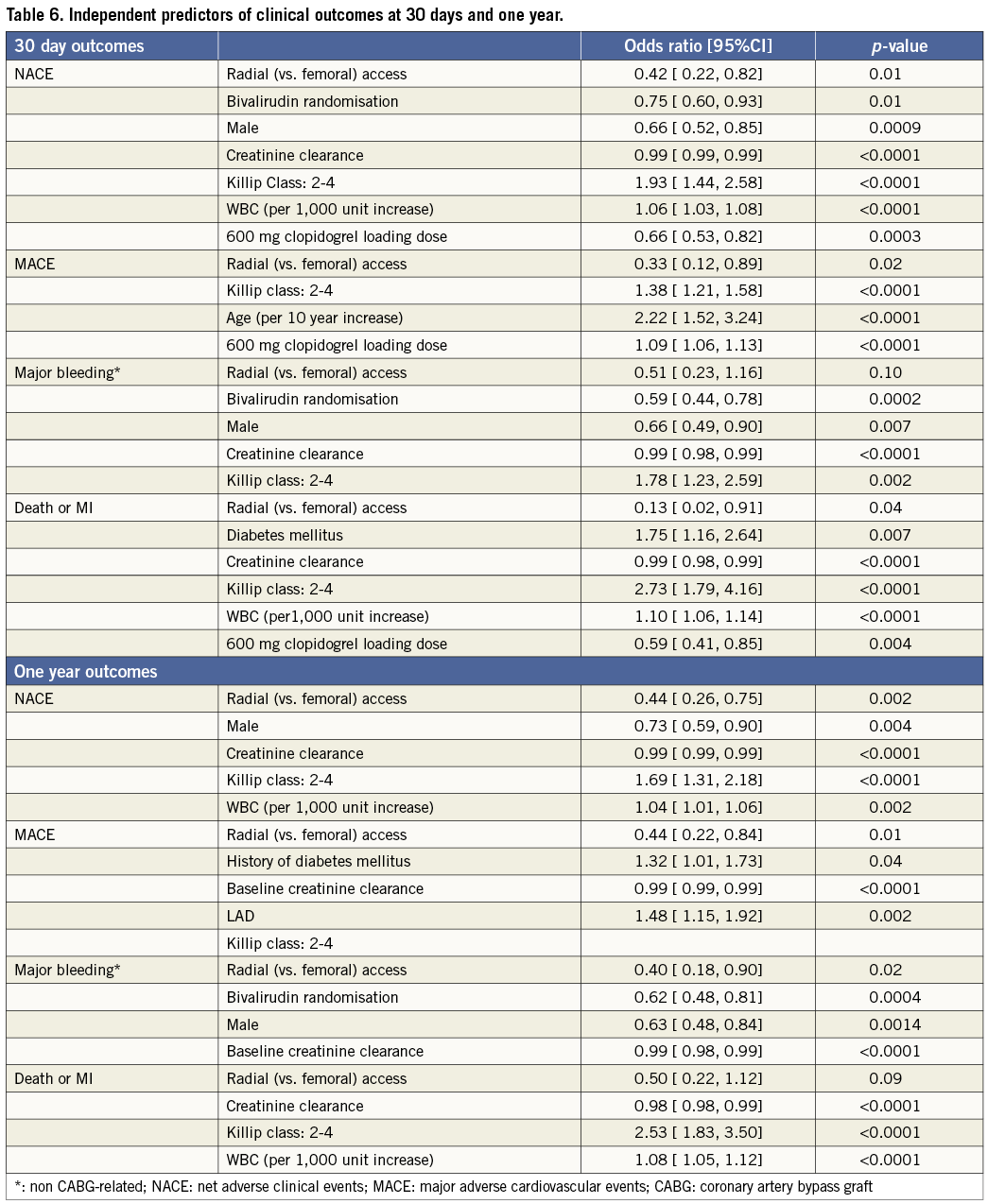
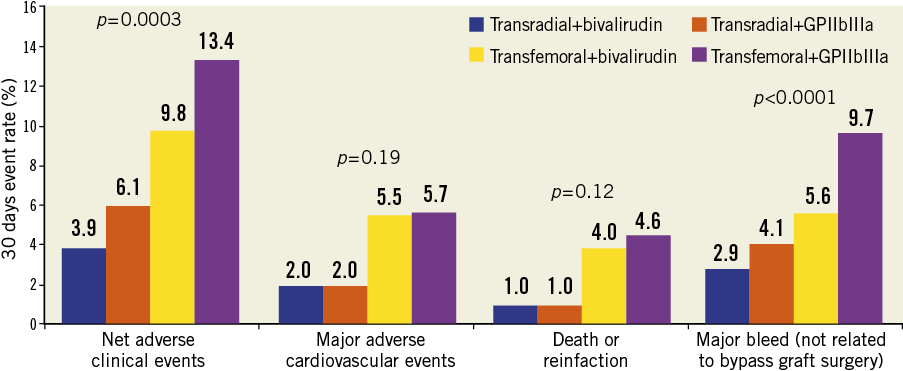
Figure 3. Clinical outcomes according to access site and randomised antithrombotic regimen at 30 days.
Discussion
The present study represents the first large-scale multicentre report examining the impact of TR vs. TF access on 30-day and one-year clinical outcomes in patients with STEMI undergoing primary PCI in the era of contemporary antithrombotic therapies. The principal findings of the present analysis are: 1) Compared with the TF approach, the TR approach was associated with lower rates of composite death or reinfarction, non CABG-related major bleeding, MACE and NACE at 30-day and one-year follow-up; 2) After adjusting for possible confounders, the TR approach remained an independent predictor of event-free survival at 30-day and one-year follow-up; 3) The lowest rates of adverse events were observed in patients treated with bivalirudin monotherapy in whom PCI was performed with TR access; 4) Nonetheless, TR access was used in only 6% of patients undergoing primary PCI for STEMI in this large multicentre international trial.
Nine prior studies comparing TR versus TF access in STEMI have been published14,22-30. In the largest prospective randomised trial, 149 STEMI patients were randomised to either the TR (77 patients) or the TF approach (72 patients25). In this small study the TR approach achieved similar rates of reperfusion and in-hospital MACE as TF access. In the largest single-centre, observational study published to date in patients with STEMI, Hetherington et al observed similar procedural success, in-hospital MACE and time to discharge between the TR approach (571 patients) and the TF approach (480 patients)28. In a recent meta-analysis including 12 studies and 3,324 patients, use of TR access in primary PCI was associated with a decrease in the risk of major bleeding, MACE and death at short-term follow-up35.
All of these prior studies were limited by either the small number of patients24-27,29, the heterogeneity of the population studied (combination of rescue and primary PCI24,26), the absence of unified definitions for major outcomes14,22-27, and/or short-term follow-up (in-hospital or 30 days14,22-24,26-29). Moreover, only heparin and GPIIb/IIIa inhibitors were used in these studies30, this being a limitation because the use of the direct thrombin inhibitor bivalirudin during primary PCI has been shown to reduce major bleeding and death in STEMI2,7. The present study is consistent with the results of these prior reports and is the first to report a significant decrease in both ischaemic and bleeding events with the TR approach in patients with STEMI undergoing primary PCI in the era of contemporary antithrombotic regimens (including bivalirudin).
In HORIZONS-AMI, TR compared to TF access was associated with a 55% reduction in the rate of non CABG-related major bleeding at 30 days, due principally to a significant reduction in the occurrence of access site-related large haematomas (≥5 cm). Overt access site-related bleeding and retroperitoneal haematomas also occurred only with the TF approach. Indeed, there were no access site-related bleeds among the 200 patients with STEMI undergoing primary PCI with TR access in this study. The fact that TR compared to TF access “only” reduced major bleeding by 55% is explained by the fact that not all major bleeds are access site related. From a recent analysis of 302,152 PCI procedures, among 7,328 (2.4%) patients with major bleeds, only 38% occurred at the percutaneous entry site (12.8% of which were retroperitoneal haematomas13).
Prior studies have shown that access site haematomas requiring transfusion are an independent predictor of one-year mortality36, and other studies have demonstrated a strong association between major bleeding complications and increased cardiac mortality37. Consistent with the present report, prior studies have demonstrated that TR access can be performed in the vast majority of patients undergoing PCI, and may prevent up to 50% of post-procedural bleeding complications13. The reduction in major access site bleeding complications with the TR approach (especially retroperitoneal haematomas) would be expected to result in fewer transfusions, less complications from haemodynamic compromise, and a reduced need to discontinue life-saving medications, such as aspirin and thienopyridines. These and other benefits might be expected to result in improved survival. However, with only 200 patients undergoing primary PCI via the TR route, the present study was underpowered to demonstrate improved survival with TR compared to TF access. The present study did, however, show a reduction in the risk of 30-day and one-year composite ischaemic endpoints when a TR approach was used.
Recently, the large-scale randomised RIVAL trial was published, demonstrating in 7,021 patients with acute coronary syndromes that TR and TF access resulted in similar rates of the 30-day composite measure of death, MI, stroke or non CABG-related major bleeding (the primary powered endpoint)20. A significant interaction was present, however, between access site and the presence of STEMI vs. non-STEMI, such that the primary endpoint net adverse event rates were reduced in STEMI patients treated with TR access (p-value for interaction=0.025). Interpretation of positive subgroup findings from a negative trial must be made with extreme caution (if at all). Nonetheless, the results from the present study demonstrating reduced rates of MACE with TR access is consistent with these findings. However, whereas both death alone and composite death, reinfarction or stroke were reduced in STEMI patients randomised to TR access in the RIVAL trial, non CABG-related major bleeding was not. While differences in definitions or ascertainment methods may explain why major bleeding was reduced with TR compared to TF access in the present and prior studies but not in RIVAL, only a definitive randomised trial of TR vs. TF access in patients with STEMI will clarify the relative outcomes of these differing approaches. Finally, bivalirudin was the foundation anticoagulant in only 3% of patients in RIVAL. In HORIZONS-AMI, the magnitude of the reduction in non CABG-related major bleeding with TR compared to TF access was more evident in patients treated with heparin and GP IIb/IIIa inhibitors than with bivalirudin. Furthermore, the lowest absolute rates of events were observed in patients treated with bivalirudin and TR access. These findings emphasise the synergy between technique and adjunct pharmacotherapy if bleeding complications are to be minimised, and warrant including bivalirudin in future randomised trials of TR vs. TF access in STEMI.
Some studies have suggested that the TR approach may be associated with longer fluoroscopy times, even in experienced hands18,20,38,39. In contrast, we observed similar procedural and fluoroscopy times. Nonetheless, TR access was associated with prolonged duration from cathlab arrival to angiography, and greater door-to-balloon times, which could be deleterious in patients with STEMI. These findings reinforce the need for an adequately powered randomised trial of TR vs. TF access in STEMI.
The HORIZONS-AMI protocol recommended selection of access site per operator discretion. Despite this provision (and the fact that the benefits of TR access in reducing bleeding were widely known during the recruitment phase of this trial), TR access was used in only 6% of patients, mainly in Europe. Potential explanations for why TR access is rarely used (at least in STEMI) include concerns regarding anatomic variants or difficult vascular access which can slow door-to-balloon time; reduced guide catheter support; need for venous access and/or haemodynamic support devices; concerns related to the success rate being reduced; lack of training or experience of the operator or staff; and the learning curve required to achieve TR expertise. Nonetheless, some studies have suggested that the advantages of the TR approach compared to the TF approach are even more evident in patients with STEMI than in stable patients20,21, likely due to the more aggressive antithrombotic regimens used and the higher risk of bleeding. In this regard a recent meta-analysis reported an absolute 3.1% reduction in major bleeding with the TR compared to the TF approach in primary PCI for STEMI, vs. a 1.8% reduction in elective PCI19.
Given the fact that the prognosis in STEMI is highly dependent on ischaemic and procedural time, operators must be well trained in the TR approach before using this technique in primary PCI. Current recommendations state that 80 to 100 TR PCI procedures are needed for an operator to achieve procedural time and success rates comparable to the TF approach40,41. Furthermore, operators should be aware that some patients are not ideal candidates for TR intervention (e.g., those with Killip IV class presentation, insufficiency of the ulnopalmar arches, and saphenous vein graft lesions). Careful patient selection is therefore of paramount importance. In contrast, even the selective use of TR access would be expected to benefit patients with high body mass index (BMI) or severe peripheral vascular disease, which warrants that all operators receive adequate training in this technique.
Several important limitations of the present analysis should be discussed. This study was an observational post hoc analysis from the HORIZONS-AMI trial, not powered to assess radial vs. femoral outcomes. Differences were present between the TR and TF groups at baseline, which may have impacted the results. For example, the lower rate of TIMI-3 flow with the TF compared to the TR route may be due to the greater proportion of thrombotic lesions in the TF group. The crossover rate from TR to TF was not recorded in this study and the current analysis was performed using the actual access site used to complete the PCI. In the RIVAL trial, 7.0% of TR patients had failed access required crossover to the TF route20, which may prolong door-to-balloon times. Sheath sizes were not recorded and may have been larger in TF patients. The experience of the operator performing TR or TF access was not known, which may affect outcomes, especially with TR PCI20. Finally, potential unmeasured confounders may be present which cannot be adjusted by multivariable analysis, a limitation compounded by the modest size of the TR group. The results of this report should therefore be considered hypothesis-generating, especially given the imbalance in the use of TR and TF access. Prospective, randomised trials are needed to further evaluate the clinical impact of radial vs. femoral approaches in patients with STEMI undergoing primary PCI, especially in patients treated with bivalirudin.
Conflict of interest statement
Dr. Lansky has received research grants from The Medicines Company, Cordis, Boston Scientific, Medtronic, and Abbott. Dr. Witzenbichler has received lecture honoraria from Boston Scientific and The Medicines Company. Dr. Mockel has received lecture honoraria from The Medicines Company. Dr. Guagliumi has served as a consultant at Boston Scientific and Volcano, and has received lecture honoraria from Boston Scientific, Medtrocin, Lightlab, Labcoat, and receiving grant support from Medtronic and Boston Scientific. Dr. Dudek has received lecture fees from Nycomed. Martin P. Fahy is employed by the Cardiovascular Research Foundation. Dr. George Dangas has received lecture honoraria from Medicines Company and Boston Scientific. Dr. Mehran has received consulting fees from Eli Lilly and Astra Zeneca and lecture honoraria from Sanofi Aventis, Cordis, Boston Scientific, and The Medicines Company. Dr. Stone is a consultant for Abbott Vascular, Boston Scientific, Medtronic and The Medicines Company. The other authors have no conflict of interest to declare.

