Abstract
Aims: The transradial approach (TRA) for percutaneous coronary intervention (PCI) recently emerged as a safer vascular access with a similar rate of MACE but a lower success rate requiring crossover to another approach when compared to the transfemoral approach (TFA).
Methods and results: In our hospital the introduction of the TRA in November 2003 resulted in a progressive decline of TFA use. Over the five years of conversion to TRA, from 2002 (100% TFA) to 2007 (98% TRA), major adverse cardiac events (MACE) and all in-hospital vascular and bleeding events, related or not to vascular access, were prospectively collected to assess performances of each approach in the specific setting of PCI (percutaneous coronary interventions). Data of 1,928 TFA and 1,672 TRA for a total of 3,600 consecutive PCI procedures are reported. PCI success rate was unchanged by TRA (96.1% versus 95.3% for TFA, NS). TRA was associated with a reduction in the rate of post-PCI myocardial infarction (2.3% versus 3.6% for TFA, p=0.023) and with a significant reduction of MACE (3.8% versus 5.2% for TFA, p=0.041). TRA use was also associated with a marked reduction of blood transfusion and surgery for post-PCI bleeding (0.2% versus 1.5% for TFA, p<0.001), despite more frequent prescription of downstream glycoprotein IIb/IIIa inhibitors (23.7% versus 7.4% for TFA, p<0.001). Thus, TRA resulted in a rapid and significant reduction of all major in-hospital adverse events, cardiac as well as non-cardiac, pooled in a “Net Adverse Clinical Event (NACE) index” of non-desirable events: death, myocardial infarction, stroke, urgent CABG surgery, surgery for bleeding and vascular events and blood transfusion. Such events occurred in 4.1% of TRA (n=69) as compared to 7% of TFA (n=134) (p<0.001), accounting for a 41% relative reduction of this NACE index by TRA. By multivariate analysis, TRA was related to a better in-hospital outcome (OR 0.64, 95% confidence interval [CI] 0.47-0.87; p=0.005).
Conclusions: TRA for PCI provides the same success rate as TFA but significantly reduces post hoc related complications.
Abbreviations
CAD: coronary artery disease
MACE: major adverse cardiovascular events
NSTEMI : non-ST-elevation myocardial infarction
PCI: percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
TFA: transfemoral approach
TRA: transradial approach
Introduction
Percutaneous coronary interventions (PCI) may require potent anticoagulants, particularly in the setting of high-risk acute coronary syndromes.
The femoral artery is the most commonly used vascular entry site for PCI. Not surprisingly, in relation to the level of anticoagulation obtained, haemorrhagic complications related to the access site do occur. The true incidence is difficult to appreciate, in part due to lack of standardised definitions1. In recent studies challenging new antithrombotic regimens, these adverse events ranged from 3% to 5%2-6.
Blood transfusions can no longer be considered as a benign issue, and have recently been linked to an increased mortality at one year7-12. Furthermore, the fear of vascular haemorrhage could prevent physicians from fully endorsing recommended antithrombotic regimens, thus potentially exposing their patients to more frequent major adverse cardiac events (MACE).
When successfully placed, vascular closure devices lead to rapid mobilisation: their use reduces the time required to achieve haemostasis and this can lessen bleeding rates but at the expense of device-related complications. To date, they have not been shown to decrease bleeding problems substantially13-19.
Transfemoral access (TFA) was challenged by the transradial approach (TRA) as the entry site for coronary angiography by Campeau as early as 198920, and a few years later by Kiemeneij for PCI21,22.
TRA was introduced into our hospital at the end of 2003 by a first “radialist”, with the addition of a second one at the end of 2004. The move to TRA for the “femoralist” operators was carried out on an unforced basis and was gradual: this resulted in a progressive shift from an all-femoral access (2003 and earlier) to an all-radial access (2006 and thereafter) cardiac catheterisation laboratory.
The aim of the present study was to assess the impact of this change in terms of procedural success and MACE.
Methods
Procedural, clinical and laboratory data were prospectively entered in a dedicated PCI database directly at the time of the procedure or during the following days, using the hospital’s electronic medical record. QCA analysis was performed at the time of catheterisation. Since 2005, owing to the local TRA development, trained personnel prospectively tracked all vascular-related events occurring before hospital discharge.
The transition TFA-TRA occurred mainly during the years 2004-2005 but we extracted PCI data from 2002 to 2007. We excluded 1,551 “one day” patients (30%): their PCI were performed by external consultants and they were sent back to their referring hospital a few hours after successful PCI, precluding an adequate follow-up of complications. Nevertheless, the same access switch occurred for them (71% of PCI performed by TFA in 2004 followed by 65 % by TRA in 2007). These patients were often those with favourable clinical characteristics and with an immediate uneventful procedure.
The access switch was supervised by the two “radialist” operators who initiated TRA locally (2004) and in 2005 started to study the success rate when both radial arteries had to be attempted first before shifting to TFA23. For the other operators, access choice was completely free. The patient was orally informed by the operator of the preferred vascular access before the procedure (but no written informed consent was required, the two techniques being largely used and validated worldwide).
After TRA PCI, sheath removal was immediate, using the TR Band™ device (Terumo, Tokyo, Japan). In the case of femoral procedure, vascular closure devices were freely used; otherwise, sheath removal and manual compression were recommended as soon as possible and were under the direct responsibility of the operator. After femoral sheath removal, mobilisation was mostly delayed for at least two to three hours according to the vascular access management strategy chosen by the operator.
About 80% of our PCI were carried out as ad hoc procedures using 500 mg i.v. bolus aspirin and i.v. unfractionated heparin (75 to 100 IU/kg). Clopidogrel (300 mg loading dose) or ticlopidine (150 mg loading dose) was given after stenting. Use of GP IIb/IIIa receptor inhibitors was unrestricted and, typically, they were continued for a maximum of 12 hrs after successful PCI. Electrocardiogram, cardiac enzymes, haemogram and blood creatinine were systematically controlled the day after PCI. Post-PCI myocardial infarctions (MI) were defined as Q or non-Q-wave depending on the presence of a new Q-wave associated with positive cardiac markers. CK or CK-MB enzymes of at least three times the laboratory’s upper normal limit were considered as a marker of myocardial infarction. Haemorrhagic events were analysed as vascular or non-vascular related. Only blood product transfusions not related to cardiac surgery were analysed.
For ad hoc angioplasty, fluoroscopy time and volume of contrast required by the angioplasty itself were recorded. As regulated in the country (Belgium) during the study period, mainly bare metal stents were used, except for diabetic patients and in case of in-stent restenosis where drug-eluting stents were used. The Leaman scoring system was used to grade the CAD severity24, both before and after the angioplasty attempt, thus providing an index of revascularisation. Success, MACE and vascular or bleeding events were routinely reviewed and censored by one investigator (ES). Hospital deaths were reported as total mortality.
Successful angioplasty was defined as post-intervention residual stenosis of less than 30% or successful stenting of all the attempted coronary lesions, and success was defined as partial when at least one attempt on one stenosis had failed.
We reported in-hospital MACE separately and as a classic “composite index” that includes any occurrence of death, MI, stroke or emergency cardiac surgery. We then pooled this “composite index” and each vascular or haemorrhagic event that required either blood transfusion or surgery in a Net Adverse Clinical Event (NACE) index. The authors are solely responsible for the design and conduct of this study, all study analyses, drafting and editing of the paper.
Statistical analysis
Numerical parameters were expressed as mean±standard deviation and were compared between TRA and TFA groups by the Wilcoxon rank sum test. Categorical and ordinal variables were compared by chi-square or Cochran tests, respectively. Multivariate analysis used logistic regression with backward selection of variables by likelihood ratio test. All statistical tests are two-tailed, and a p-value of less than 0.05 was considered to be statistically significant. Analysis was performed using SPSS 15.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
Figure 1 depicts the progressive transition from an all-femoral access to an all-radial access catheterisation laboratory. The shift occurred mainly in 2005. A total of 3,600 PCI were analysed, 1,928 by femoral approach and 1,672 by TRA.
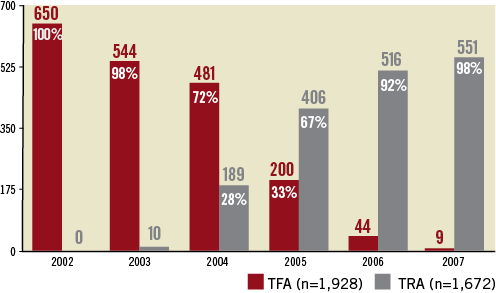
Figure 1. Conversion from TFA to TRA for 3,600 PCIs.
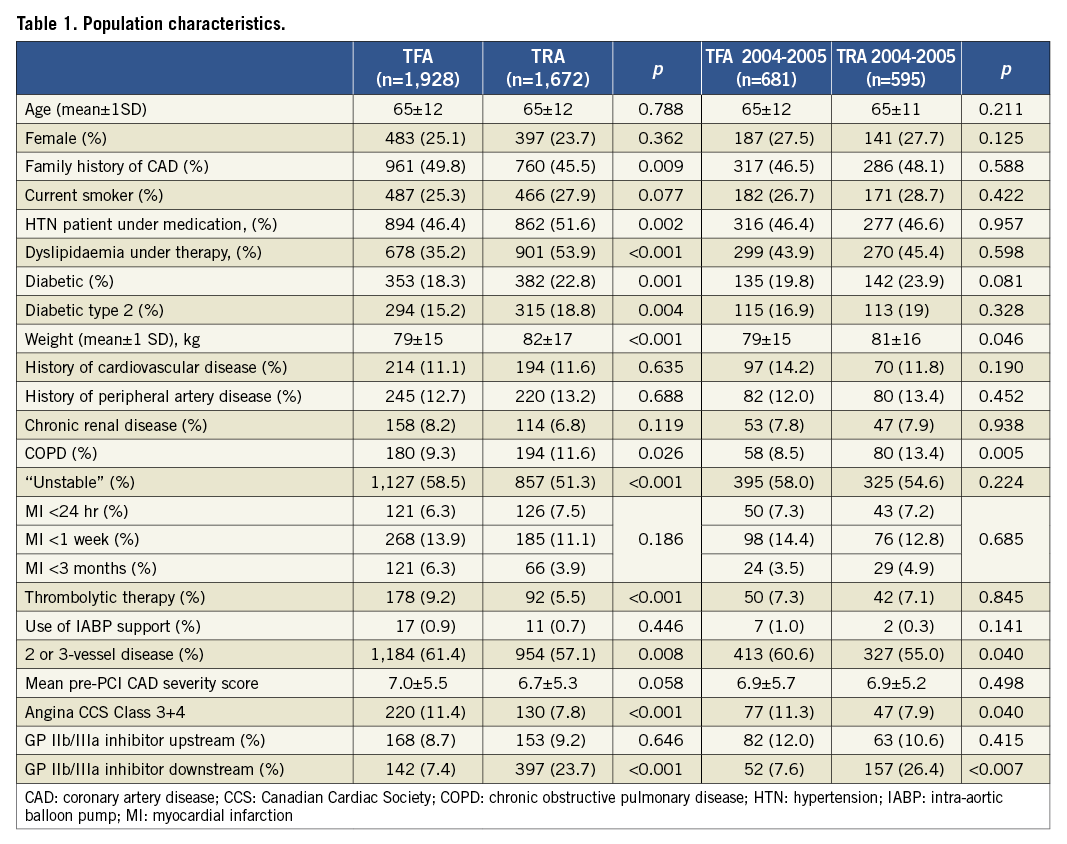
Table 1 depicts the population characteristics: from 2002 to 2007, we observed a progression of the number of hypertensive patients, greater use of statins, but also more diabetic and more overweight patients; fewer patients received thrombolytic therapy for STEMI. Mean age remained stable, as did the proportion of female and post-MI patients stratified as acute (<24 hr), subacute (first week) or recent (<3 months). CAD severity as assessed by the CAD score was not statistically different for the two cohorts (p=0.058).
Downstream use of GP IIb/IIIa receptor inhibitors grew with TRA (23.7% versus 7.4% for TFA, p<0.001) and resulted in a doubling of the total use of this medication (32.9% versus 16.1%) over the study period. The use of downstream GP IIb/IIIa receptor inhibitors was unbiased by the patient’s age and resulted in a 31.5% prescription for patients in their seventies and 11.4% for octogenarians.
Regarding the PCI success (first endpoint), the study showed no statistical difference (Figure 2). The PCI succeeded for 96.1% TRA and for 95.3% TFA (NS). CAD severity score after PCI was statistically lower after TRA (2.96±4.20 versus TFA 3.38±4.52, p=0.002), reflecting a better revascularisation.
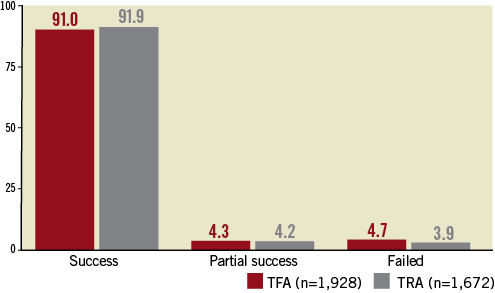
Figure 2. PCI success rate (%).
For the TRA cohort, median fluoroscopy time was 42 seconds longer (504 seconds versus 546 seconds for TRA, p=0.036) and the amount of contrast media 10 ml higher (mean 226±99 ml versus 217±108 ml for TFA, median 210 ml for TRA versus 200 ml for TFA, p<0.001) .
Twenty-two TRA patients (1.3%) required crossover to TFA (sometimes after a failed bilateral radial attempt). We already reported 6.8% use of the contralateral radial artery23.
In-hospital adverse events are reported in Table 2. The death rate was 1.5% for TRA versus 1.9% for TFA (NS). Urgent CABG surgery was required for 0.2% TRA versus 0.3% for TFA (NS). A definite stroke occurred after 0.3% TRA versus 0.2% for TFA (NS). A higher rate of post-PCI MI was observed in the TFA group: 3.6% (n=70) versus 2.3% (n=39) for TRA (p=0.023), mainly due to a reduced rate of NSTEMI: 3.06% (n=59) versus 2.01% (n=33) (p=0.039). This lower rate of NSTEMI with TRA significantly influences the classic “composite index” combining death, MI, urgent CABG surgery and stroke: 3.8% (n=63) of such events for TRA compared to 5.2% (n=100) for TFA (p=0.041).
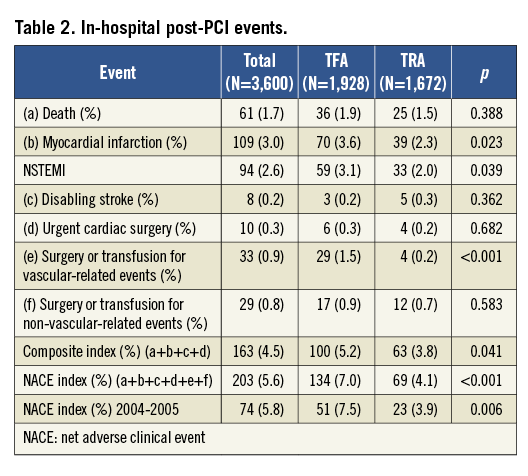
Blood transfusion or vascular surgery was reported in only 0.2% of patients (n=4) after a TRA PCI and in 1.5% (n=29) of patients after TFA PCI (p<0.001).Vascular closure devices accounted for 7.7% (n=149) of TFA procedures. Haemorrhages not related to vascular access sites and requiring surgery or blood transfusion occurred after 0.7% (n=12) of TRA procedures versus 0.9% (n=17) for those performed by TFA (NS), despite twice the number of patients receiving GP IIb/IIIa receptor inhibitors in the TRA group (Figure 3).
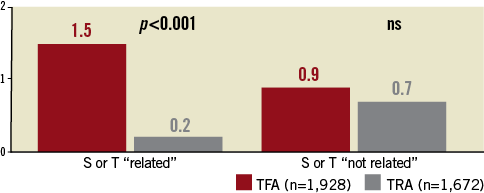
Figure 3. Post-PCI haemorrhagic events requiring surgery or transfusion (S or T) related or not related to the vascular access site (%).
Therefore, TRA is also, and logically, associated with a significant reduction in the net adverse clinical events (NACE) index regrouping all major in-hospital adverse events, namely death, MI, stroke, urgent CABG surgery, any surgery for bleeding and non-CABG surgery-related transfusion: 4.13% (n=69) of events for TRA versus 6.95% (n=134) of events after TFA driving an absolute reduction of 2.82% (40% relative reduction) (p<0.001) (Figure 4). Figure 5 illustrates the temporal evolution of the NACE index from 2002 to 2007: a significant (p=0.048) and rapid decline occurred in 2004-2005 (the transition period).
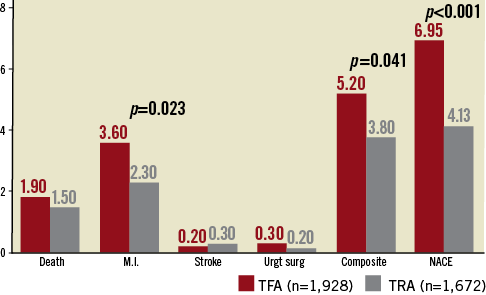
Figure 4. Post-PCI major clinical events (%). Composite index: death or myocardial infarction (MI) or disabling stroke or urgent cardiac surgery (Urgt surg); NACE: net adverse clinical event: composite index+surgery or transfusion for vascular haemorrhagic events
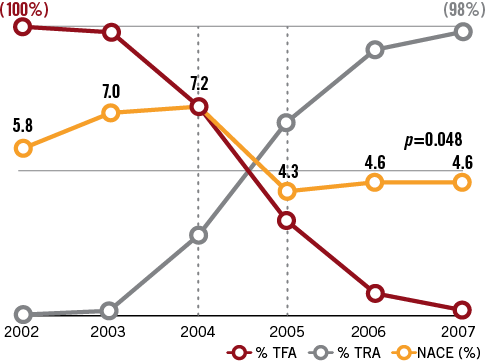
Figure 5. NACE (2002 to 2007) and use of TFA vs. TRA for 3600 PCI. NACE: death or myocardial infarction, or disabling stroke or urgent cardiac surgery or surgery or transfusion for vascular as non-vascular-related haemorrhagic events; TFA: transfemoral access; TRA: transradial access
During the critical two years of the vascular access shift (2004-2005) the NACE index also declined significantly, 23 events occurring in the TRA group (3.9%) versus 51 events (7.5%) in the TFA group, p=0.006 (Table 2).
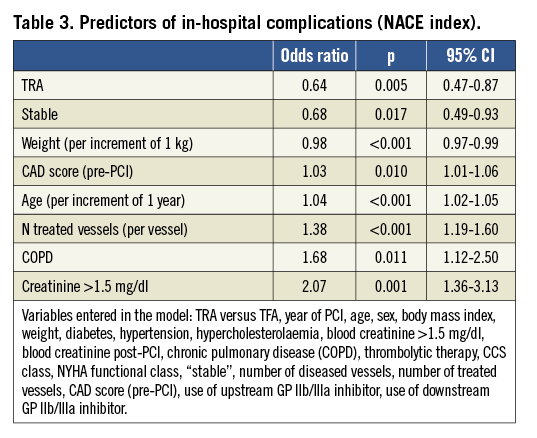
Using this NACE index, a multivariate logistical regression analysis (Table 3) shows that the use of TRA remains the best predictor of a better in-hospital outcome (odds ratio=0.64, 95% CI: 0.47-0.87, p=0.005). On the contrary, older age (per increment of one year) (p<0.001), low body weight (per increment of one kilogram) (p<0.001), baseline creatinine ≥1.5 mg/dl (p=0.001), increasing number of treated vessels (per vessel) (p<0.001), pre-PCI score of CAD severity (p=0.010), COPD (p=0.011) and unstable condition (p=0.017) negatively affected the in-hospital prognosis.
Discussion
TRA PCI is reported to be a safer approach in terms of vascular-related complications without affecting the MACE rate25-33. Though recently questioned23,34-36, the success rate of this approach has been reported as inferior, requiring conversion to TFA25,28,37,38. The recent RIVAL randomised study39 concluded that “radial access reduced major vascular complications compared with femoral access, with similar success rates. The effectiveness of radial access might be linked to expertise and volume”.
Our study illustrates a local conversion from a strategy of “by default TFA” to “by default TRA” for PCI. As pointed out by the RIVAL study, PCI’s success rate remained unchanged. It is perhaps in part due to the rapid high volume of TRA procedures performed by each operator. Crossover to TFA was low (1.3%), due to the systematic use of the contralateral radial artery access instead of going immediately to the groin for a TFA: this strategy equalises the catheterisation success rate for both approaches as reported previously by our team23.
The study shows the relatively short period of time (two years) required for the shift, and illustrates the size of the move: in the last year analysed, 98% of PCI were performed through TRA with a very rare crossover to femoral access. Considering the fact that two of the four operators were “true” radial beginners, radiation exposure was not remarkably increased (42 seconds), nor the volume of contrast agent used (10 ml). It is possible that the observed rapid conversion was the result of some mentoring by the two radialists.
The conversion resulted locally in a reduction of post-PCI in-hospital major adverse events. Decreased rates of bleeding, transfusion and vascular events25,27, as well as MACE26 and even mortality7,40,41, have been reported with TRA. Bleeding and transfusions are linked to a worse prognosis at one-year follow-up9,42. Thus, the observed reduction of vascular events requiring surgery or transfusion (0.2% TRA vs. 1.5% TFA) is consistent with previous reports25,28. Our 1.5% rate for TFA is well within the accepted range despite the recent RIVAL study showing an unusual “low rate of major bleeding at the level of the access site compared with previous studies and attributed to the great operator experience for the two techniques”. Furthermore, we also observed a decline in the rate of post-PCI NSTEMI. This observation should be read in the context of a single-centre and non-randomised study: these results could be driven by definite study population differences and/or time-related changes. It is nevertheless tempting to link this reduced NSTEMI rate with the growing use of downstream GP IIb/IIIa inhibitors in the TRA cohort. These data support the hypothesis that diminishing the risk of bleeding with systematic use of TRA for PCI permits the full benefit of potent anticoagulants in terms of reduction of ischaemic events38. It is possible that the change in the perceived balance of the risk versus the benefit of such potent antithrombotic medications as GP IIb/IIIa inhibitors had influenced the operator’s decision to use these therapies. It is also possible that the benefit of TRA could arise mainly in situations requiring a high level of anticoagulation, as seen with high-risk acute coronary syndrome. This could explain why STEMI patients derived the most important benefit from TRA in the RIVAL study. It is unknown if the use of bivalirudin, given its more favourable safety profile in the setting of TFA PCI for ACS5, could equalise TRA advantages. On the other hand, the use of such anticoagulants for TRA could further improve safety by reducing the occurrence of non-vascular bleeding.
The multivariate analysis of post-PCI adverse events is in accordance with the expected factors affecting the prognosis for a general PCI population: a stable condition results in a better outcome, while a worse outcome is expected for unstable patients, older patients, patients with more advanced CAD or with a comorbidity (COPD, peripheral arterial disease or some renal impairment). The positive prognosis influence for the factor “weight” may be regarded as the consequence of the well-known “obesity paradox” added to the same worry-free and unaffected TRA safety performance for obese patients.
Although not randomised, this study of 3,600 PCI has its own merits. First, no previous study had explored further the impact of a radical change in vascular access site choice for PCI and its consequences on MACE at the level of an entire and important catheterisation laboratory from a tertiary hospital. Second, starting from a complete TFA and moving to an almost entirely TRA approach, the bias linked to patient selection was rapidly removed: operator propensity for selecting cases for TRA was progressively abolished between 2004 and 2005 for the two “femoralists”, and was avoided for the two “radialist” operators who selected TRA for their patients by study protocol23. Furthermore, because a “real world” population is being considered, the study is more likely to reflect true life. Of course, differences mainly related to the study period emerged in the two cohorts of patients: these differences may have contributed to the better outcome of the most recent population. Nevertheless, we do not expect major changes in the severity of the disease to occur in so short a period of time. In particular, the rapid reduction of the NACE index observed during the key period of the shift (Figure 5) is a good argument for a causal relationship. Furthermore, the major characteristics for cardiac disease severity and comorbidities look quite well matched, particularly looking at years 2004 and 2005. Many confounding factors are reduced in this single-centre study with the use of the same material, the same operators, the same post hoc instructions and follow-up, and the same nursing staff.
Finally, the TRA favourable difference cannot be explained by the performance of the two “radialist” operators: being entirely “radial” since the beginning, their practice cannot be dissociated from the total TRA results.
Study limitation
Although the data presented have been prospectively collected, this is a retrospective analysis study. Due to the serial and temporal nature of these observations, some findings could be explained by newer therapeutic strategies, better operator experience or some changes in the study population. In the same way, the rapidity of the conversion to a full TRA cathlab may have been driven by the study protocol started in 2005 by two operators and forcing them to use TRA as a default, first intention vascular access.
Conclusion
In summary, this large study of 3,600 PCI illustrates conversion from TFA to TRA for PCI. The transition occurred rapidly and safely, without impacting on the PCI success rate. Locally, this move resulted in a reduction of a net adverse clinical events index, regrouping all major in-hospital adverse events, namely death, MI, stroke, urgent CABG surgery, any surgery for bleeding and, finally, transfusion. The study provides another piece of evidence of the added value of TRA for PCI.
Conflict of interest statement
The authors have no conflict of interest to declare.

