
Abstract
Aims: The aim of this study was to evaluate whether a staged in-hospital complete revascularisation strategy increases the risk of serious bleeding events in patients with ST-segment elevation myocardial infarction (STEMI) and multivessel disease.
Methods and results: The DANAMI-3-PRIMULTI trial investigated whether a staged in-hospital complete revascularisation strategy improved outcome in patients with STEMI and multivessel disease. In this substudy, we investigated potential bleeding complications related to a second in-hospital procedure. Bleedings were assessed using BARC and TIMI criteria. Six hundred and twenty-seven (627) patients were randomised 1:1 to either PCI of the infarct-related artery (IRA) only (n=313) or complete revascularisation during a staged procedure before discharge (n=314). We found no significant difference in TIMI major+minor bleedings related to the primary PCI. There were neither major nor minor bleedings in relation to the second procedure in the complete revascularisation arm. There were significantly more in-hospital minimal+medical attention bleedings in the group randomised to complete revascularisation (61.5% vs. 49.5% in the IRA-PCI only group, p=0.003), but no difference in admission time or one-year mortality (2.2% complete revascularisation-group vs. 2.6% IRA-PCI only group, p=0.8).
Conclusions: In multivessel diseased STEMI patients, a staged complete in-hospital revascularisation strategy or any second in-hospital procedure did not result in an increase in serious bleeding events.
Abbreviations
ACS: acute coronary syndrome
BARC: Bleeding Academic Research Consortium
DANAMI-3-PRIMULTI The Third DANish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction: PRImary PCI in MULTIvessel Disease
FFR: fractional flow reserve
IRA: infarct-related artery
MVD-STEMI patients: multivessel diseased STEMI patients
PCI: percutaneous coronary intervention
STEMI: ST-segment elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
Introduction
The risk of events related to thrombosis in acute coronary syndrome (ACS), such as death and myocardial infarction, has decreased, resulting in a shift in focus towards procedure-related bleeding events, which are known to have a serious association with short- and long-term mortality1-3.
Approximately 40-50% of patients presenting with ST-segment elevation myocardial infarction (STEMI) have multivessel disease, and these patients are known to have a poorer prognosis4,5. The American guidelines concerning revascularisation of multivessel diseased STEMI patients (MVD-STEMI patients) have just been updated in the light of recent clinical trials suggesting a beneficial effect of complete revascularisation6-9. The clinical trials on the basis of which the guidelines have been modified had different approaches on when to perform the complete revascularisation procedure, and it remains debated whether it should be carried out during the index procedure or as a staged procedure. In two large meta-analyses, acute multivessel PCI (during the index procedure) was associated with the highest mortality, whereas staged multivessel PCI (later during the index hospitalisation or within one month) was associated with the lowest mortality10,11.
In the largest of the trials, the DANAMI-3-PRIMULTI trial, MVD-STEMI patients were randomised to either conventional treatment with acute PCI of the IRA only, or to a staged, in-hospital complete revascularisation strategy with acute PCI of the IRA and a second in-hospital procedure with fractional flow reserve (FFR)-guided full revascularisation12.
In this substudy we investigated the potential bleeding complications in relation to a second in-hospital procedure in the DANAMI-3-PRIMULTI trial.
Methods
STUDY DESIGN, PARTICIPANTS AND RANDOMISATION
The DANAMI-3-PRIMULTI trial is a part of the DANAMI-3 trial program which encompasses three trials (Figure 1). The study is conducted at four large primary PCI (PPCI) centres in Denmark. The study protocol has previously been described in detail12. Briefly, patients with chest pain of <12 hours’ duration and ST-segment elevation in a minimum of two contiguous leads were randomised to PPCI performed with a deferred strategy of stent implantation, ischaemic post-conditioning or conventional treatment. In the PRIMULTI part of the trial, patients with multivessel disease and initial successful PPCI were randomised, after the PPCI, to either complete FFR-guided revascularisation plus optimal medical therapy or to optimal medical therapy alone (Figure 1). Complete revascularisation was achieved by a staged in-hospital procedure strategy (Figure 1). After a mean follow-up time of 27 months, the primary endpoint (a composite of all-cause mortality, non-fatal reinfarction and ischaemia-driven revascularisation of non-IRA lesions) had occurred in 22% in the IRA-PCI only group, and in 13% in the complete revascularisation group (p=0.0004). The difference was mainly due to a higher rate of unplanned ischaemia-driven revascularisations in the IRA-PCI only group.
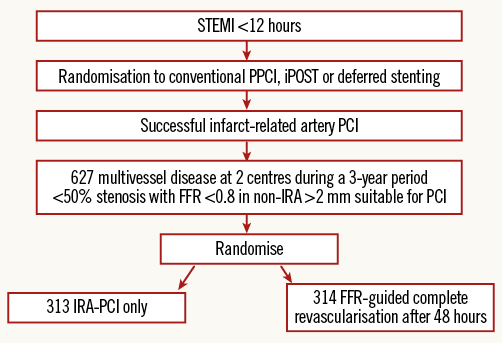
Figure 1. Randomisation flow chart of the DANAMI-3-PRIMULTI trial. FFR: fractional flow reserve; iPOST: ischaemic post-conditioning; IRA: infarct-related artery; PPCI: primary percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction
PROCEDURES
In patients randomised to complete revascularisation, the intention was to perform the additional PCI procedure 48 hours after the PPCI, during the index hospitalisation. Patients randomised to IRA-PCI only could have a scheduled second in-hospital procedure due to randomisation to deferred stent implantation (Figure 1). All additional patient management, including follow-up, rehabilitation, anticoagulant and antithrombotic regimens took place at local hospitals where the treating physicians were not part of the study staff.
STUDY OUTCOMES
The endpoints in this study were bleeding incidents of any kind and severity, assessed according to the TIMI and BARC bleeding criteria3. All patients’ hospital records, haemoglobin levels and notes written by doctors and nurses within one year after admission were reviewed. If a patient had several bleeding incidents, all events were assessed and registered. All deaths were specifically evaluated with regard to being bleeding-related. Event data were recorded electronically and stored at the Clinical Trial Unit of Rigshospitalet, Copenhagen.
STATISTICAL ANALYSIS
The analyses were performed on an intention-to-treat basis. Time-to-event analyses and bleeding probabilities were displayed using cumulative incidence methodology with relevant first bleeding as the event of interest and death as a competing risk. Differences between groups in time-to-event endpoints were assessed with Gray’s test. Comparisons of bleeding events between the two groups were assessed by chi-square or Fisher’s exact test. Variables predictive of bleeding events were assessed with the Cox proportional hazards model test. Baseline variables were compared using chi-square tests for categorical variables, t-tests for continuous variables with normal distributions, and Wilcoxon rank-sum tests for continuous variables with non-normal distributions. A p-value <0.05 was considered significant. SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
ETHICS
The DANAMI-3-PRIMULTI trial protocol (ClinicalTrials.gov number: NCT01960933) complied with the Declaration of Helsinki, and received ethics committee approval according to local regulations as well as approval from the Danish Data Protection Agency (2007-41-1667). All patients provided written informed consent.
Results
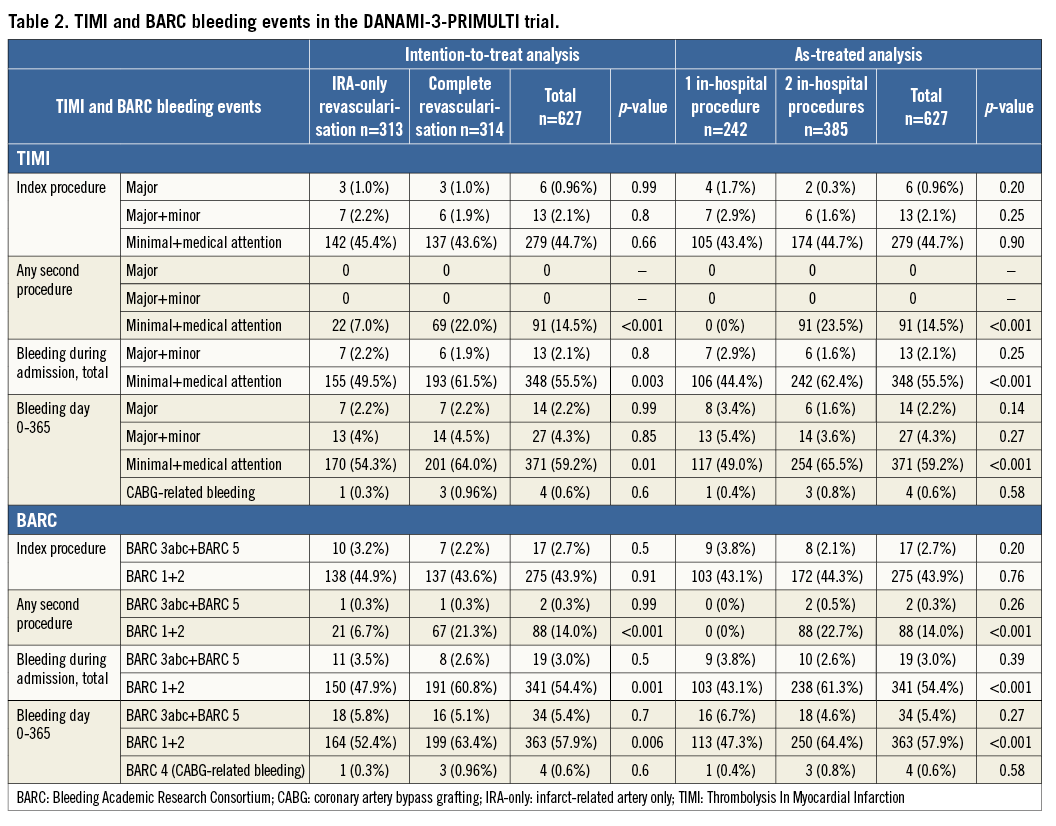
From March 2011 to February 2014, 627 patients were randomised in the DANAMI-3-PRIMULTI trial (Figure 1). Three hundred and fourteen patients were allocated to undergo an FFR-guided second invasive procedure in non-IRA lesion(s) in a median of two days after the primary PCI, while 313 were assigned to no further invasive treatment. Baseline characteristics were well balanced between the two groups (Table 1). A total of 305 (97%) of the patients randomised to full revascularisation received a second in-hospital procedure while 82 (26%) of the patients randomised to IRA-PCI only received a second in-hospital procedure12. Second in-hospital procedures in the IRA-PCI only group occurred due to large thrombus burden, control of stent implantation, randomisation to deferred stenting or post-procedural angina (two patients where a non-IRA lesion was treated). Incidences of bleeding events are displayed in Table 2 and time-to-event analyses are illustrated in Figure 2 and Figure 3.
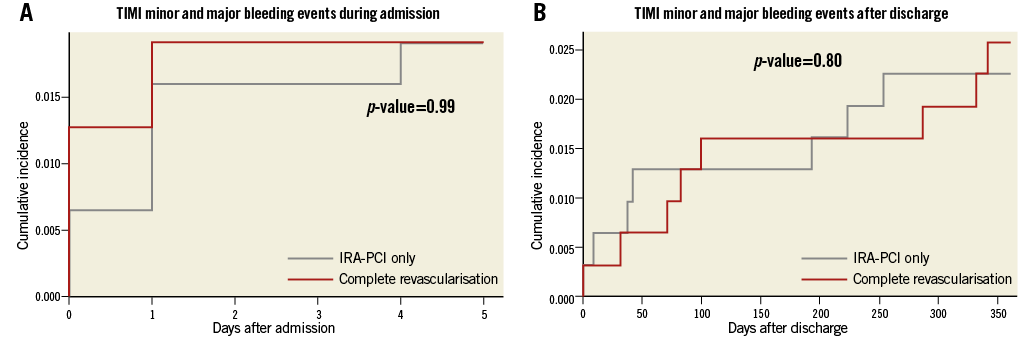
Figure 2. Minor and major bleeding events in the DANAMI-3-PRIMULTI trial. A) Cumulative incidence of TIMI minor and major bleedings during admission (day 0-5). B) Cumulative incidence of TIMI minor and major bleedings after discharge (day 6-365). IRA-PCI only: index-related artery PCI only
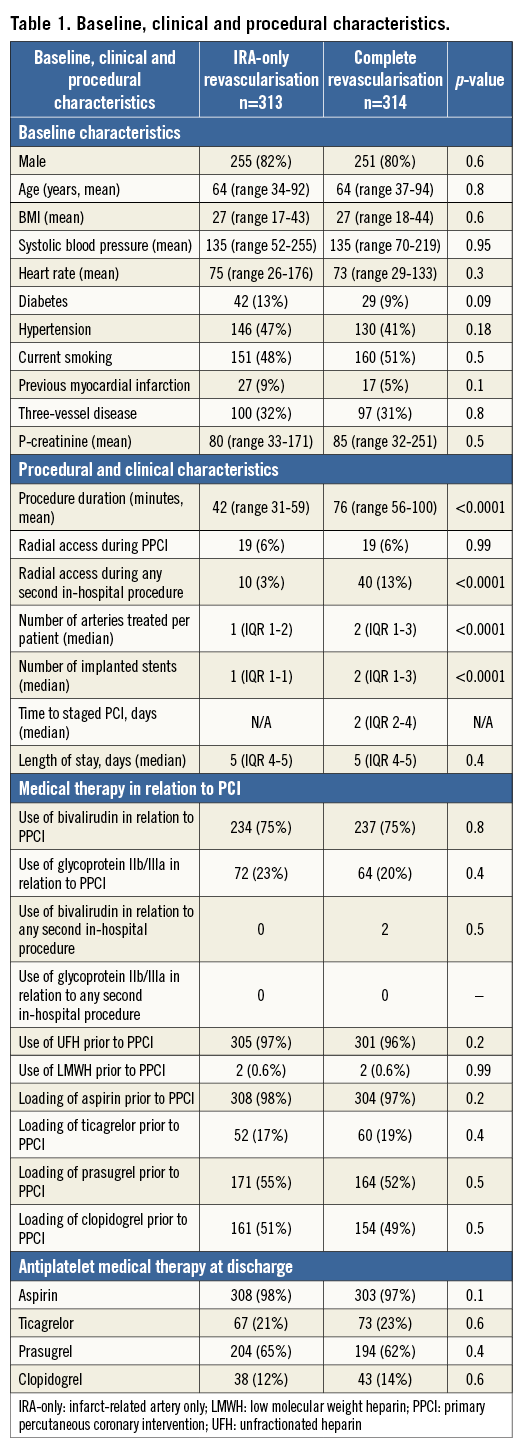
Figure 3. Minimal and medical attention bleeding events in the DANAMI-3-PRIMULTI trial. A) Cumulative incidence of TIMI minimal and medical attention bleedings during admission (day 0-5). B) Cumulative incidence of TIMI minimal and medical attention bleedings after discharge (day 6-365). IRA-PCI only: index-related artery PCI only
As expected, we found no significant difference in bleeding events in relation to the index procedure between the two groups (Table 2). There were no patients in the entire study population who had either a TIMI minor or major bleeding related to any second in-hospital procedure (Table 2)3. Furthermore, there was no significant difference in minor+major bleedings during admission or after discharge (Table 2, Figure 2), or in one-year mortality in the groups (2.2% complete revascularisation vs. 2.6% IRA-PCI only, p=0.8). In total, 4.3% of the 627 DANAMI-3-PRIMULTI patients experienced a TIMI minor or major bleeding within 365 days after admission (Table 2).
In-hospital TIMI minor+major bleedings consisted of access-site bleedings (55%), retroperitoneal bleedings (15%) and bleedings from the gastrointestinal tract (15%), while both of the bleeding-related deaths were due to haemopericardium (15%). No patients received blood transfusions between procedures. In the group randomised to complete revascularisation, 13% had the second procedure performed via radial access, and 10% via the contralateral femoral artery. The remainder had the second procedure through the same access as the index procedure.
There were significantly more in-hospital minimal+medical attention bleedings in the complete revascularisation group, due to a higher number of small bleedings related to the second procedure (Table 2, Figure 3)3. In total, 96 of the 627 patients experienced a TIMI minimal or medical attention bleeding in relation to a second in-hospital procedure, 90 of which were access-site bleedings (24 [8%] in the IRA-PCI only group vs. 66 [21%] in the complete revascularisation group, p<0.0001). We found no difference in less severe bleedings after discharge (Table 2, Figure 3).
Due to the fact that 26% in the IRA-only group received a second in-hospital procedure, we performed additional as-treated analyses. As expected, the only significant difference was found in minimal+medical attention bleedings during admission due to the bleedings occurring in relation to any second procedure in the group receiving two procedures (Table 2). There was no difference in minor+major bleedings during admission or after discharge, or minimal+medical attention bleedings after discharge (Table 2).
In a multivariate Cox proportional hazards model adjusted for relevant variables (gender, age, BMI, serum creatinine at admission, systolic blood pressure [SBP] at admission, three-vessel disease, randomisation to full revascularisation, radial access during PPCI, use of glycoprotein IIb/IIIa inhibitors [GP IIb/IIIai] during PPCI, use of bivalirudin during PPCI and pretreatment with either clopidogrel, ticagrelor or prasugrel), only male gender was predictive (protective) of in-hospital minor or major bleeding (HR 0.15 [CI: 0.04-0.63], p=0.01). Variables predictive of in-hospital minimal+medical attention bleedings were SBP >191 mmHg at admission (HR 2.26 [CI: 1.17-4.35] p=0.02), use of GP IIb/IIIai during PPCI (HR 1.60 [CI: 1.20-2.14] p=0.001) and randomisation to complete revascularisation (HR 1.31 [CI: 1.05-1.67] p=0.02). After discharge, a variable predictive of minor or major bleeding was male gender (HR 0.19 [CI: 0.06-0.69], p=0.01), while no variables were found to be predictive of minimal+medical attention bleedings. Among patients receiving two in-hospital procedures, the use of GP IIb/IIIai did not increase bleeding risk (data not shown). Eptifibatide was the most commonly used GP IIb/IIIai in the present study, hence only three patients were treated with abciximab. None of them had minor or major bleedings.
TIMI minimal+medical attention bleedings were not associated with an increased risk of subsequent TIMI minor and major bleedings or increased one-year mortality (data not shown). TIMI in-hospital minor and major bleedings, however, were strongly associated with one-year mortality with a hazard ratio of 14.00 (CI: 4-50, p<0.0001).
Discussion
This is the first study to investigate thoroughly possible bleeding complications related to a staged in-hospital complete revascularisation strategy in MVD-STEMI patients in a randomised clinical trial. No serious bleeding events were found in relation to a second in-hospital procedure. Furthermore, the incidence of one-year major bleedings in the DANAMI-3-PRIMULTI population was similar to results reported in previous studies investigating bleeding events in ACS7,13. Previous, retrospective registry studies on MVD-STEMI patients have suggested the same; however, the methodological challenges of these studies have made it difficult to interpret the results and apply them to daily practice14,15.
Though we found an increased risk of in-hospital less severe bleedings in patients receiving any second in-hospital procedure, the incidence of procedure-related less severe bleedings was lower in the second than in the first procedure. This may reflect a more calm and elective setting, and the fact that practically no patients received bivalirudin or GP IIb/IIIai, there was a higher use of radial access and the patients were not under the heavy influence of UFH loading before arterial puncture. Prior studies have reported the prognostic impact of access-site vs. non-access-site bleedings and small bleedings according to other bleeding classifications, but to our knowledge there are no existing data associating TIMI minimal+medical attention bleedings to increased mortality16,17. In our study, even a drop of blood on the band aid over the access site was registered and assessed in severity. Thus, many of the less severe events registered in relation to procedure number 2 were very discreet access-site haemorrhages, as would be expected when perforating an artery. The fact that the complete revascularisation group, which had a significantly higher incidence of in-hospital TIMI minimal+medical attention, had neither a prolonged length of stay during primary admission, a higher incidence of serious bleedings nor an increased one-year mortality, suggests that these bleeding events are in fact less severe and do not cause a need for prolonged observation nor do they affect one-year mortality. Hence, we interpret them as clinically insignificant. The as-treated analyses provide the exact same results. The only difference in bleeding incidences among patients receiving one in-hospital procedure vs. two in-hospital procedures was found in minimal+medical attention bleedings during admission. This strengthens our notion that it appears safe to receive a second in-hospital procedure.
The rate of radial access was low in this study. This is due to the fact that the study was conducted from 2011 to 2014, before the focus on lower risks related to radial access. However, the fact that the majority of severe in-hospital bleedings were access-site bleedings is in concert with findings in other studies1,17.
Traditional predictors of bleeding such as age, serum creatinine and especially radial access were not found to be significant in the present study. This may be due to the low bleeding rates and the size of the study population.
When comparing bleeding events assessed in accordance with the BARC criteria in comparison to the TIMI criteria, we found a slightly higher number of BARC serious bleedings than TIMI serious bleedings, but still no significant difference between the two groups.
The DANAMI-3-PRIMULTI trial showed that it is safe and perhaps even beneficial, with regard to a combined ischaemic endpoint, to perform complete staged revascularisation during the index hospitalisation, compared with IRA-PCI only. Our study suggests that this strategy does not increase the risk of serious bleedings.
Limitations
Notes from general practitioners were not available. Thus, less severe bleeding events treated only by general practitioners after discharge are likely to be underreported. TIMI minimal/BARC1 events included after discharge are cases reported by patients retrospectively, in a hospital setting. Only three patients received abciximab, the GP IIb/IIIai with the longest-acting antithrombotic effect, during admission, making it difficult to investigate its bleeding-related implications in staged procedures. Nearly all patients in the study (97%) (Table 1) were treated with UFH prior to PPCI. Furthermore, 75% of patients received bivalirudin during/after PPCI, usually after the ACT was measured. In the HEAT-PPCI study, a bivalirudin-based strategy was associated with an increased bleeding risk while the BRIGHT study showed opposite findings. Hence, whether a strict and ACT-based regimen of heparin as monotherapy combined with GP IIb/IIIai as bail-out would have increased or decreased bleedings in the present study is speculative18,19. Though no serious bleedings occurred in relation to any second in-hospital procedure, suggesting that the second procedure is safe, one cannot exclude the possibility that a significant, increased risk of bleeding would have been found if the study population had been larger.
The DANAMI-3 trial was an all-comer study; however, during the screening process, 66 patients were excluded due to haemorrhagic diathesis, known coagulopathy, severe comorbidity or severe renal insufficiency12. These patients traditionally carry a higher bleeding risk and are excluded from most clinical trials. Hence, the overall bleeding incidence in the DANAMI-3 trial could be lower than in a true unselected population.
Conclusion
We found no serious bleeding events related to the second in-hospital complete revascularisation procedure, or any second in-hospital procedure, in STEMI patients with multivessel disease. We did find an increased risk of less severe bleedings in the group receiving complete in-hospital revascularisation, but these bleedings were not associated with prolonged admission or increased one-year mortality.
| Impact on daily practice The optimal timing of the complete revascularisation procedure in MVD-STEMI patients remains a topic of debate. This study suggests that there is no increased risk of serious bleedings when performing the complete revascularisation or any other second procedure as a staged in-hospital procedure. |
Acknowledgements
We would like to thank Rikke Sørensen, MD, PhD, for serving as expert reviewer regarding bleeding classifications.
Funding
This research is supported by the Danish Agency for Science, Technology and Innovation, the Danish Council for Strategic Research (EDITORS: Eastern Denmark Initiative to Improve Revascularization Strategies, grant 09-066994), The Danish Heart Foundation, The A.P. Møller Foundation and The Aase and Einar Danielsen Foundation.
Conflict of interest statement
The authors have no conflicts of interest to declare.

