Abstract
Aims: To determine the relation between thrombus aspiration (TA) and/or intra-lesion (IL) abciximab with pre-stent Thrombolysis In Myocardial Infarction (TIMI) flow grade and infarct size (IS) in patients with ST-elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI).
Methods and results: The INFUSE-AMI trial randomised 452 patients with anterior STEMI to IL abciximab vs. no abciximab, and to manual TA vs. no TA. The primary endpoint was cMRI-determined IS at 30 days. Patients were classified according to pre-stent TIMI flow. Complete data were available in 290 patients - 68 (25.2%) with pre-stent TIMI 0/1 flow, 47 (17.4%) with TIMI 2 flow and 175 (57.4%) with TIMI 3 flow. Patients with pre-stent TIMI 3 flow had significantly lower IS (15.5% [4.6, 21.8] vs. 22.6% [14.7, 28.0] for TIMI 2 vs. 19.5 [14.4, 27.8] for TIMI 0/1, p<0.0001) and fewer 30-day clinical events (p=0.03). Patients receiving TA with or without IL abciximab had the highest rate of pre-stent TIMI 3 flow (p<0.0001) and patients receiving both had the smallest IS (14.7% vs. 17.3% for the other three groups, p=0.03).
Conclusions: Optimisation of coronary flow prior to stent implantation may reduce infarct size and clinical events in STEMI patients undergoing primary PCI.
Introduction
Primary percutaneous coronary intervention (PCI) is the preferred strategy for reperfusion in patients with acute ST-elevation myocardial infarction (STEMI) because it results in better infarct artery patency and myocardial salvage than fibrinolysis or conservative management1,2. Despite important advances in technique, equipment and adjunctive pharmacology, suboptimal reperfusion continues to exist in ~20% of patients, presumably because of residual thrombus and distal embolisation during PCI3. Thrombus aspiration (TA)4 and intracoronary (IC) abciximab5 are two of the most studied interventions to prevent these complications.
In the Intracoronary Abciximab and Aspiration Thrombectomy in Patients With Large Anterior Myocardial Infarction (INFUSE-AMI) randomised clinical trial (NCT00976521), randomisation to intra-lesion (IL) abciximab resulted in a significant reduction in median infarct size (IS), measured by contrast magnetic resonance imaging (cMRI) at 30 days, compared to no abciximab – 15.1% vs. 17.9% of left ventricular mass, p=0.03. However, this reduction in IS was not accompanied by significant improvement in markers of reperfusion, such as final Thrombolysis In Myocardial Infarction (TIMI) flow grade, myocardial blush grade (MBG) or ST-elevation resolution. In contrast, thrombus aspiration had no significant effect on IS.
Thus, we hypothesised that the effect of IL abciximab on infarct size may be enhanced by improvement in pre-stent coronary flow.
Methods
INFUSE-AMI has been described in detail6,7. In brief, 452 patients with anterior STEMI and anticipated symptom onset to reperfusion time of <5 hrs undergoing primary PCI with bivalirudin anticoagulation were randomised in a 2x2 factorial design to IL abciximab delivered locally via the ClearWayTM RX catheter (Atrium Medical, Hudson, NH, USA) vs. no abciximab, and to thrombus aspiration using the Export® catheter (Medtronic, Minneapolis, MN, USA) vs. no thrombus aspiration. The primary endpoint of the study was IS, measured at 30 days by cMRI and expressed as % of left ventricular (LV) mass in patients assigned to IL abciximab vs. no abciximab, pooled across strata of thrombus aspiration. The secondary major endpoint was IS in patients assigned to aspiration vs. no aspiration, pooled across strata of IL abciximab. All patients received aspirin, and clopidogrel or prasugrel.
All angiograms were reviewed in detail at the Cardiovascular Research Foundation Angiography Core Laboratory. Specifically, qualitative and quantitative analysis (QCA) was performed for TIMI flow grade, MBG, corrected TIMI frame counts (cTFC) at baseline, pre-stent, and final angiogram, using standard definitions8,9. MBG was assessed according to the semi-quantitative densitometric method, which evaluates the maximal intensity of contrast penetrating the infarct zone, in comparison to unaffected territories10. Thrombus area was measured at the lesion site at the same time points. TIMI flow grade 3 was considered successful epicardial reperfusion, and MBG grade 2/3 was considered successful microcirculatory (myocardial) reperfusion. The sequence of utilisation of IL abciximab and/or TA was recorded and the patients were divided into four groups based on which intervention was used. IS was assessed at the Cardiovascular Research Foundation MRI Core Laboratory. Quantitative analysis was performed using FDA approved ReportCard 4.0 software. Left ventricular (LV) volumes were determined by manually tracing the endocardial borders, excluding the papillary muscles, at end-diastole and end-systole on all short-axis cine-CMR images. LV mass (the area between epicardial and endocardial borders) and infarct area (the white area within the black myocardium) were segmented on the DE-CMR images11. Percent infarct mass was calculated as infarct mass divided by total myocardial mass.
We first analysed the relationship between pre-stent TIMI flow (divided into 0/1, 2 and 3) and IS, and then assessed the frequency of the TIMI flow categories in the four intervention groups and the respective IS in each group. We included in this study all patients who had complete angiographic and cMRI data.
STATISTICAL ANALYSIS
Continuous variables are presented as mean with standard deviation or median with interquartile range and were compared with the Wilcoxon rank-sum test. Categorical variables are presented as proportions and were compared with chi-square or Fisher’s exact test. IS was not distributed normally and is represented only as median values. Significance level was set at 0.05. All analyses were performed with SAS 9.2 (SAS Inc., Cary, NC, USA).
Results
Of the 452 patients enrolled in INFUSE-AMI, 367 had pre-stent QCA data, 352 had cMRI data at 30 days (primary endpoint) and 290 patients had both – representing this study population. Pre-stent QCA data were missing in 85 patients because the investigators did not mark in the procedure log the timing of the angiogram relative to use of ClearWay or aspiration catheter.
There were 68 patients (25.2%) with pre-stent TIMI 0/1 flow, 47 (17.4%) with TIMI 2 flow and 175 (57.4%) with TIMI 3 flow. The baseline characteristics of the three groups, based on pre-stent TIMI flow grade, are shown in Table 1. There were no significant differences among the three groups. The procedural characteristics of the three groups are shown in Table 2. Two thirds of the patients with pre-stent TIMI 3 flow had TIMI 0/1 flow at baseline, suggesting marked improvement in epicardial flow before stent placement in a majority of patients. Only two patients (1.1%) of those with pre-stent TIMI 3 flow developed no-reflow (TIMI 0/1) at final angiogram. Importantly, thrombus area decreased by ~50% in all patients from baseline to pre-stent angiogram, and thrombus was completely absent in more than half the patients with pre-stent TIMI 3 flow.
Infarct size, expressed as % of LV mass at 30 days, in the patients with TIMI 3 flow prior to stent was significantly lower than in patients with less optimal flow (Figure 1). Similarly, IS was lower in patients with MBG 2/3 prior to stent, compared with those with MBG 0/1 (Figure 2). When thrombus was still present at the lesion site before stent implantation, IS was greater than when thrombus was absent (Figure 3).
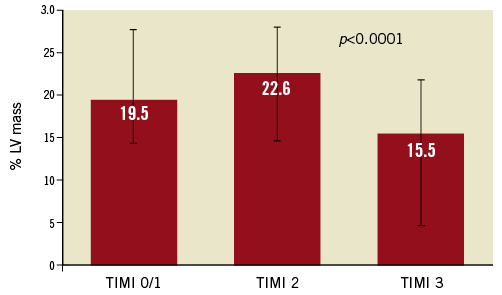
Figure 1. Infarct size (% of left ventricular [LV] mass) according to pre-stent TIMI flow. TIMI: Thrombolysis In Myocardial Infarction flow grade
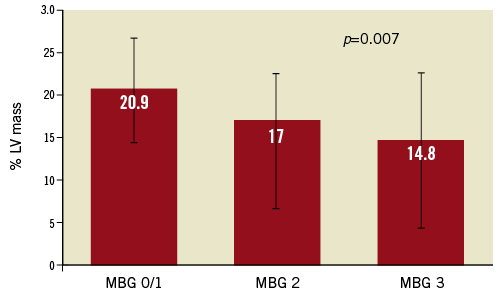
Figure 2. Infarct size (% of left ventricular [LV] mass) according to pre-stent myocardial blush grade (MBG).
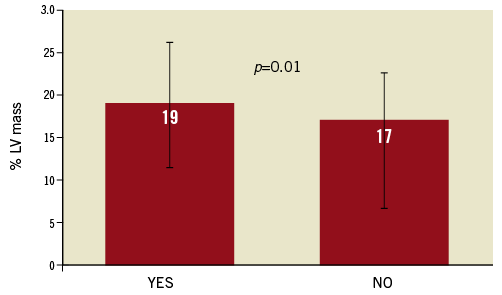
Figure 3. Infarct size (% of left ventricular [LV] mass) according to pre-stent thrombus presence.
In order to relate utilisation of IL abciximab, thrombus aspiration and their combination to IS, we tabulated the frequency of TIMI 3 flow at baseline, before stent and at final angiogram in the four permutations of the two randomisation strata. As shown in Figure 4, patients receiving aspiration with or without IL abciximab had higher rates of TIMI 3 flow before stent than those treated with abciximab alone or neither intervention (p<0.001) despite lack of differences at baseline or at final angiogram. Similar results (not shown) were noted with respect to MBG 2/3 or presence of thrombus pre-stent. Consistent with these results, IS was lower in patients receiving the combination of both interventions, compared with the other three groups (p=0.03, Figure 5). It is notable that, in 91% of the patients receiving both interventions, TA was performed before IL abciximab was administered.
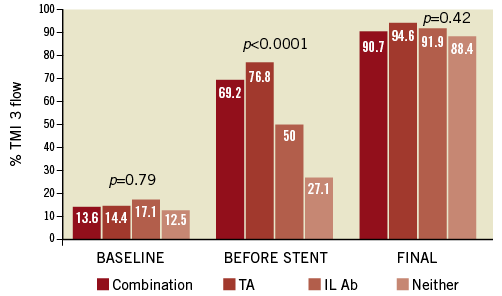
Figure 4. Rates of TIMI 3 flow according to use of thrombus aspiration (TA) and intra-lesion abciximab (IL Ab). TIMI: Thrombolysis In Myocardial Infarction flow grade
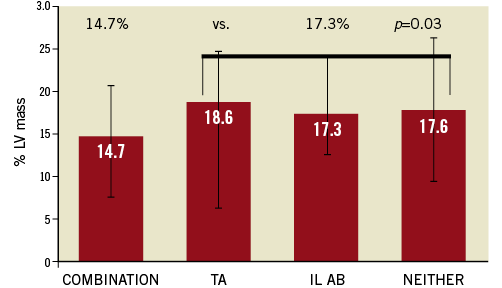
Figure 5. Infarct size (% of left ventricular [LV] mass) according to use of thrombus aspiration (TA) and intra-lesion abciximab (IL Ab).
The incidence of death, myocardial infarction, ischaemia-driven revascularisation, stroke or (new) heart failure requiring hospitalisation at 30 days was significantly lower for pre-stent TIMI 3 flow patients – 1.7% vs. 8.5% for TIMI 2 and 7.4% for TIMI 0/1, p=0.03.
Discussion
The principal findings of our study can be summarised as follows:
1) Despite lack of difference in markers of reperfusion among the four groups of patients with various permutations of thrombus aspiration and intra-lesion administration of abciximab at baseline and final angiogram, epicardial flow was improved in patients receiving TA with or without IL abciximab prior to stent implantation.
2) Pre-stent TIMI 3 flow was linked to smaller infarct size, assessed by cMRI at 30 days, and fewer adverse clinical events at the same time point.
In INFUSE-AMI, IL abciximab was associated with a smaller infarct size, while thrombus aspiration had no effect on IS. The 16% reduction in IS with IL abciximab was not accompanied by commensurate improvements in rates of final TIMI 3 flow, MBG 2/3 or ST-segment resolution at 60 minutes after PCI. In an analysis of determinants of IS, we showed that IL abciximab was an independent predictor of smaller IS at 30 days (p=0.02), together with lesion location, ischaemic time, sum of ST-elevation pre-PCI, extent of CAD, MBG after PCI and complete ST-segment resolution12. The data we present in this study may provide a possible explanation for the apparently conflicting facts mentioned above, by stressing the importance of achieving optimal reperfusion just prior to stent implantation. It is notable that only five patients in this analysis (1.7%, Table 2) had worse TIMI flow before stent than at baseline, suggesting that our data present the true effect of optimising flow before stenting rather than the deleterious effects of intraprocedural thrombotic events (IPTE), associated with diminished flow and worse outcome13.
Many studies have evaluated the significance of baseline TIMI flow and outcome in STEMI patients undergoing primary PCI14-16. In an analysis combining the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) and the Harmonizing Outcomes with RevascularIZatiON and Stents in Acute Myocardial Infarction (HORIZONS AMI) trials, we found that one in six patients had TIMI 3 flow at baseline angiography17. At one year, pre-PCI TIMI 3 flow was an independent predictor of survival (hazard ratio [95% CI] = 1.65 [1.01, 2.71], p=0.046), even after correcting for final TIMI 3 flow and other important baseline and procedural characteristics. In that analysis we lacked data on IS – potentially the link between epicardial artery flow and outcome. In a small cohort of 118 patients, Ndrepepa et al showed that pre-PCI TIMI flow >1 was associated with a reduction in IS of nearly 50% (11.0% [6.1%; 23.5%] of the left ventricle in the group with TIMI flow grade <1, versus 6.0% [2.0%; 12.8%] of the left ventricle in the group with TIMI flow >1, p=0.008)18. None of these studies, though, evaluated the significance of pre-stent rather than baseline TIMI 3 flow in determining final infarct size. Furthermore, baseline TIMI flow is not amenable to modification, as it represents the interplay between the thrombus burden, duration of occlusion and intrinsic fibrinolytic response in each individual patient; in contrast, pre-stent TIMI flow can be modified by some interventions, such as TA or IL abciximab, as shown in Figure 4.
Restoration of flow may be achieved during primary PCI either spontaneously or after mechanical interventions – such as passing a guidewire, aspirating thrombus, with balloon angioplasty or with direct stenting. In some cases, there is deterioration of flow from the pre-stent to the post-stent angiogram, presumably related to distal embolisation of plaque and thrombus from the lesion, caused by the large profile of the stent delivery system, as shown initially in the Primary Angioplasty in Myocardial Infarction Trial19. Thus, removal of the substrate for distal embolisation by aspirating thrombus and causing de-aggregation of the residual thrombus with IL abciximab20 may prevent this complication of primary PCI. Indeed, thrombus aspiration has been associated in some reports with improved outcome21. Intracoronary abciximab was also shown in some studies to reduce IS compared with IV abciximab (15.1% vs. 23.4%, 35% reduction, p=0.01)11, but not in others22. In INFUSE-AMI, IL abciximab was compared with placebo, not with IV abciximab, but the combination of thrombus aspiration and IL abciximab seemed to result in the largest effect on IS – a 15% reduction compared with the other three groups combined (neither intervention, TA or IL abciximab). Furthermore, INFUSE-AMI is different from the other studies because it utilised direct intra-lesion abciximab, as compared with its intracoronary injection11,22, which results in an unpredictable dose reaching the thrombus, particularly when it is injected in the left coronary artery with its larger distribution bed.
Limitations
We recognise certain limitations of the messages derived from this analysis. This is a post hoc attempt to reconcile the lack of improvement in markers of reperfusion between baseline and final angiogram with the observed reduction in IS, and has the inherent limitations of such an approach. While this was a systematic and independent review of all angiographic data, a significant proportion of the cohort - 19% - did not have pre-stent TIMI flow data and even more patients were excluded from the analysis because of concurrent lack of cMRI data, resulting in a relatively small cohort of patients. The angiographic core laboratory could evaluate coronary flow only when recorded on film and we could not exclude the possibility that pre-stent TIMI flow did not change between the sequence available for analysis and actual stent deployment. We did not have accurate records of the interval between symptom onset and pre-stent flow evaluation. We also did not have data on administration of vasoactive substances which may have affected coronary flow. While there seemed to be a reduction in clinical events in patients with pre-stent TIMI 3 flow, we could not correct for other important covariates because of the small number of events (12 altogether). The overall differences in IS are small and their clinical relevance needs to be interpreted with caution.
Conclusion
Despite these limitations, we conclude that optimisation of coronary flow prior to stent implantation appears to confer a potential advantage with respect to final infarct size, and is associated with a possible reduction in clinical events. The combination of thrombus aspiration followed by IL abciximab achieves this goal more often than either of these interventions alone. If confirmed in other studies, these data provide the mechanistic support for recommending meticulous optimisation of infarct-artery flow prior to stent implantation in patients with STEMI undergoing primary PCI.
Conflict of interest statement
R. Mehran reports serving as consultant for Cardiva, Cordis, The Medicines Company, Regado Biosciences, and receiving research grant from Bristol-Myers Squibb/Sanofi-Aventis; C.M. Gibson received research grant support from Atrium Medical; G. Stone reports serving on the advisory boards for, and receiving honoraria from, Boston Scientific and Abbott Vascular. The other authors have no conflicts of interest to declare.

