Primary percutaneous coronary intervention (PCI) is the gold standard of treatment for ST-elevation acute myocardial infarction (STEMI)1. Although primary PCI restores optimal blood flow in the infarct-related artery (IRA), a sizeable proportion of patients experience the so-called “no-reflow” phenomenon, i.e., they continue to exhibit overt impairment of myocardial reperfusion despite successful opening of the IRA1. No-reflow occurs due to microvascular obstruction (MVO) and has a variable prevalence ranging from 5% up to 60%1. The underlying mechanisms of myocardial injury and MVO are multiple and interacting (Figure 1)1. Above all, an important determinant of MVO is distal embolisation of thrombus debris or micro-material from fissured and ruptured atheromatous plaques in the IRA going downstream during balloon dilatation or stenting. Importantly, it is associated with larger infarct size, poor functional recovery and increased long-term mortality1.
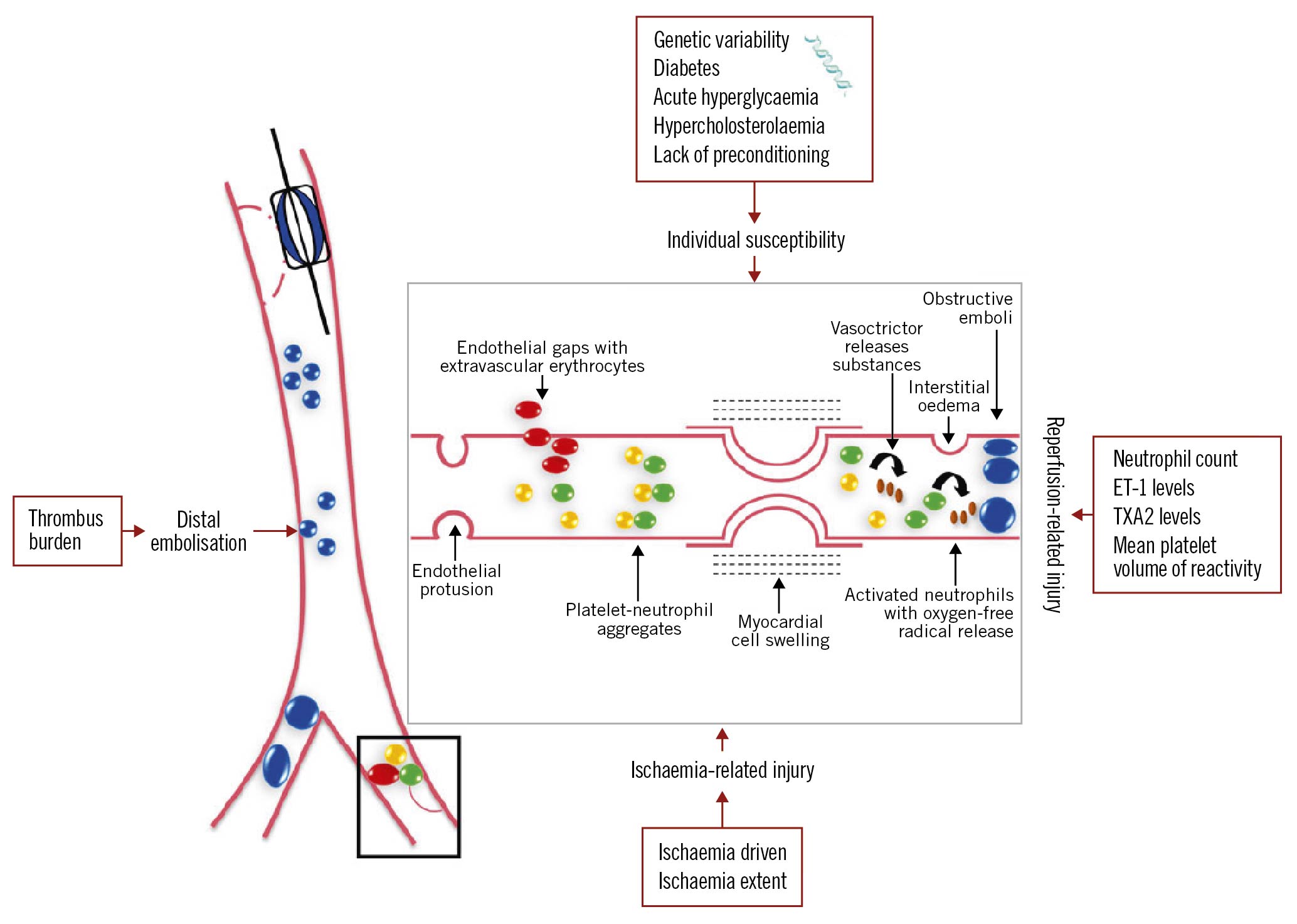
Figure 1. The four interacting mechanisms involved in the pathogenesis of coronary microvascular obstruction in humans. Ischaemic injury, reperfusion injury and distal embolisation interact with the individual susceptibility of the microcirculation to injury.
There is currently no consensus available on how best to prevent or treat no-reflow. Several intracoronary (i.c.) agents are often administered at the discretion of interventional cardiologists, despite the lack of clear evidence of their role in the management of no-reflow1. The use of low-dose fibrinolysis at the time of the primary PCI, conversely, has gained increasing attention in recent years as a potential new strategy to improve post-procedural coronary flow.
Fibrinolytic therapy is a well-recognised effective treatment for acute coronary thrombosis when PCI cannot be performed in a timely manner. However, the combination of PCI with a systematic administration of either full-dose or half-dose lytic therapy (‘facilitated PCI’) is harmful, as pre-PCI fibrinolysis improves the initial culprit artery patency but increases residual thrombus, ischaemic complications, and major bleeds, as compared with standard primary PCI.
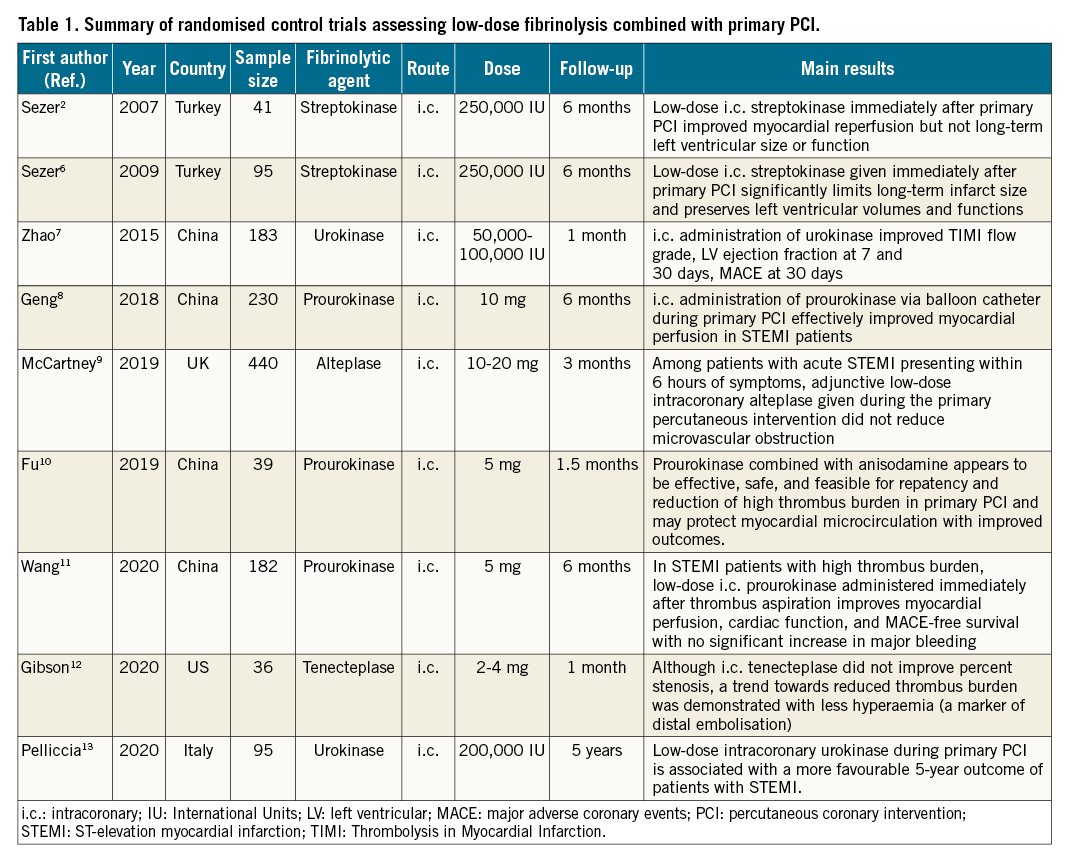
In 2007, Sezer et al performed a pilot study of forty-one patients and proposed that the i.c. infusion of low-dose streptokinase (250,000 IU) immediately after primary PCI might dissolve epicardial embolic thrombi and the microvasculature in situ thrombi, thus improving myocardial perfusion and preventing the no-reflow phenomenon2. Since the pioneering work of Sezer et al2, several investigations have assessed the role of low-dose i.c. fibrinolysis, either with streptokinase and urokinase in an earlier era, or with new-generation fibrin-specific thrombolytic agents, such as alteplase and prourokinase (Table 1). An updated meta-analysis of the 9 randomised clinical trials on low-dose fibrinolysis in primary PCI of STEMI performed to date has recently been published3. In a total of 1,341 patients, i.c. fibrinolysis significantly improved the incidence of major cardiovascular events, cardiac function, and myocardial microcirculation indicators such as the Thrombolysis In Myocardial Infarction (TIMI) flow grade and the TIMI frame count. It is noteworthy that no additional bleeding events were observed in the i.c. fibrinolysis group. The review of individual studies reveals that most trials have been able to demonstrate some favourable effects of low-dose fibrinolysis at the time of primary PCI, whereas the T-TIME (A Trial of Low-Dose Adjunctive Alteplase During Primary PCI) trial found that low-dose intracoronary alteplase compared with a placebo did not reduce MVO on cardiac magnetic resonance imaging. Overall, the pharmacologic approach was shown to be safe, with no differences in major bleeding or myocardial haemorrhage, but there were no differences in major adverse cardiac events at 1 year. A non-prespecified analysis of the T-TIME trial has shown that treatment with low-dose i.c. alteplase during primary PCI was associated with increased MVO in those STEMI patients presenting with an ischaemic time of 4 hours or more4. These findings are hypothesis-generating and raise the possibility that the effects of low-dose fibrinolysis during primary PCI might differ substantially depending upon the duration of pre-PCI myocardial ischaemia.
The pathophysiological background of the low-dose approach relates to the histologic demonstration that thrombotic material at the site of a coronary artery occlusion is often characterised by lytic and organised areas, rather than only by layered patterns of fibrin and intact platelets, erythrocytes, and granulocytes, as a consequence of a series of nonocclusive atherothrombotic events occurring in the preceding days or weeks5. The possibility exists that thrombi with different morphologies cause different types of microvascular obstructions at the time of primary PCI in patients with STEMI and, probably, require complementary therapeutic strategies. Pathological evidence, therefore, might explain why several pharmacological or mechanical strategies tested in recent years for the optimisation of primary PCI have often yielded poor results1. Indeed, one should consider that the increase in microvascular resistance typically associated with primary PCI is caused by vessel wall components, circulating blood cells, and fibrinogen, as well as by embolisation of larger thrombi with an erythrocyte-rich component5.
The rationale for the use of i.c. low-dose fibrinolysis at the time of PCI is also due to the fact that the i.c. route of administration offers several pharmacological advantages. First, a selective i.c. strategy can quickly reach a greater drug concentration at the thrombus than with systemic use, as depositing the drug at the site of arterial injury creates an intramural reservoir of drug that may increase the dissolving of the intraluminal clot1. Second, the mechanical disruption of the clot may be a further important component of site-specific fibrinolysis, as local drug administration might lead also to a spatial deformation of the thrombus, possibly due to an increase in the surface area available for fibrinolysis. Third, low-dose fibrinolysis can minimise the systemic depletion of fibrinogen, which in turn might reduce major bleeding complications. The clinical implications of these effects are that low-dose fibrin specific fibrinolytic agents given at the lesion site are associated with enhanced efficacy and safety. Indeed, a low dose of a locally delivered fibrinolytic agent is a balanced dose, high enough to induce fibrinolysis, yet low enough to limit the risk of haemorrhage.
What about the future? Three randomised, controlled, currently ongoing trials are testing the role of i.c. fibrinolysis during primary PCI (Supplementary Table 1). The results of these trials are much awaited as they may shed light on multiple unanswered questions. First, which patients might benefit the most from low-dose fibrinolysis at the time of primary PCI? It is possible that the pharmacoinvasive strategy might be particularly indicated in the subset of patients with larger thrombi, in whom the high thrombotic burden may be the major pathophysiological mechanism underlying MVO. Second, which dose, mode and timing of administration of low-dose fibrinolysis is most effective at the time of primary PCI? The available evidence suggests that any fibrinolytic agent should be administered via the i.c. route before PCI using approximately 20% of the systemic dose in order to obtain the maximum dissolution of thrombus. Last but not least, the pathogenesis of MVO in STEMI is multifactorial and therefore drug-induced thrombus dissolution cannot be the only therapeutic choice but should be coupled with other strategies aimed at limiting reperfusion damage. There is an unmet need for future studies to evaluate whether targeting both infarct size and MVO using a combination of therapies would eventually be successful in improving outcomes for this group of patients.
In conclusion, pending the results of the ongoing randomised controlled trials, the possibility exists that a future-back approach of using low-dose fibrinolytic therapy at the time of primary PCI might ameliorate either the burden of epicardial and microvascular thrombus or, eventually, the short- and long-term outcome of patients with STEMI.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

