Abstract
Aims: We assessed the impact of early infarct-related artery (IRA) recanalisation on the outcomes of patients in the recently conducted, large-scale, multicentre HORIZONS-AMI trial.
Methods and results: Of the 3,602 patients enrolled in the HORIZONS-AMI trial, 3,093 patients (85.9%) were treated with percutaneous coronary intervention (PCI) to a single artery. We analysed one-year outcomes in these patients according to the presence or absence of early IRA patency, defined as Thrombolysis in Myocardial Infarction (TIMI) 2 or 3 flow in the IRA. Baseline coronary angiography showed early IRA patency in 1,121 patients (36.2%), while 1,972 patients (63.8%) had TIMI 0 or 1 flow. The presence compared with the absence of early IRA patency was associated with better angiographic results after primary PCI with more TIMI 3 flow after PCI (93.2% vs. 82.9%, p<0.0001) and myocardial blush grade 2 or 3 (84.4% vs. 71.1%, p<0.0001). Early IRA patency was associated with lower rates of one-year mortality (2.5% vs. 3.9%, p=0.04) and definite or probable stent thrombosis (2.0% vs. 4.0%, p=0.002). In multivariable analysis, early IRA patency at baseline angiography was an independent predictor of reduced mortality at one year (HR 0.58, 95% CI: 0.36-0.98, p=0.02).
Conclusions: Early IRA patency in patients with STEMI undergoing primary PCI is associated with better TIMI flow and myocardial blush post PCI and is an independent predictor of lower one-year mortality. ClinicalTrials.gov identifier NCT00433966.
Introduction
Primary percutaneous coronary intervention (PCI) is the preferred treatment in ST-segment elevation myocardial infarction (STEMI)1,2. Previous studies have shown the benefit of spontaneous early infarct-related artery (IRA) recanalisation before primary PCI3-5. However, these studies did not incorporate contemporary therapies, including clopidogrel loading in the emergency department, glycoprotein IIb/IIIa inhibitors (GPI), bivalirudin and drug-eluting stents (DES). The objective of the present analysis was to assess the role of pre-PCI IRA patency on angiographic, electrocardiographic and clinical outcomes in patients with STEMI undergoing primary PCI with the latest innovations in STEMI care.
Methods
PATIENT POPULATION AND STUDY PROTOCOL
The HORIZONS-AMI trial was a prospective, open-label, randomised, multicentre trial comparing bivalirudin alone to unfractionated heparin (UFH) plus GPI in patients with STEMI undergoing primary PCI6,7. In brief, a total of 3,602 consecutive STEMI patients ≥18 years of age who presented within 12 hours from symptom onset were enrolled. Patients were randomised 1:1 in the emergency department after treatment with aspirin to treatment either with a combination of UFH and GPI (abciximab or eptifibatide) or bivalirudin alone. A clopidogrel loading dose of either 300 mg or 600 mg was administered prior to PCI, followed by a maintenance dose of 75 mg daily for six to 12 months. Patients eligible for stenting were randomised in 3:1 fashion either to a paclitaxel-eluting stent (DES: TAXUSTM EXPRESS; Boston Scientific, Natick, MA, USA) or to an otherwise identical, uncoated, bare metal stent (BMS: EXPRESS; Boston Scientific). For the purpose of this analysis, only patients who underwent primary PCI to the IRA without treatment of additional lesions were considered, to avoid the confounding effect of multiple lesions. They were stratified according to the presence or absence of early IRA patency as defined by Thrombolysis in Myocardial Infarction (TIMI) 2 or 3 flow (vs. 0 or 1)8 (Figure 1).
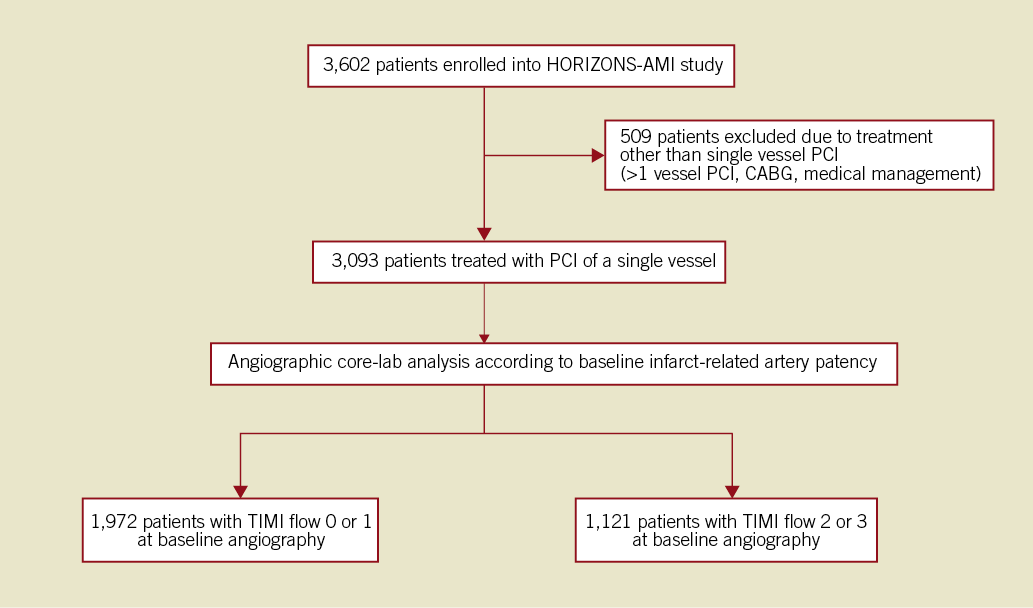
Figure 1. Study group distribution according to infarct-related artery patency at baseline angiography.
STUDY ENDPOINTS
The definitions of the study endpoints have been reported previously6,7. The primary endpoints of the current analysis were death from any cause and the cumulative rate of definite or probable stent thrombosis (according to Academic Research Consortium classification)9 at one year. Secondary endpoints were reinfarction, target vessel revascularisation for ischaemia, major bleeding, the composite endpoint of major adverse cardiovascular events (MACE, all-cause death, reinfarction, target vessel revascularisation for ischaemia or stroke) and net adverse clinical events (NACE, MACE or major bleeding not related to CABG). All endpoint events were adjudicated by an independent clinical events committee blinded to treatment assignment using original source documents. Electrocardiographic and angiographic analyses were performed at a core laboratory using validated methods by technicians who were unaware of the treatment assignments and clinical outcomes. Presence of ST-segment resolution >70% at 60 minutes after PCI was assessed by comparison to the qualifying ECG. Myocardial reperfusion before and after PCI was graded, using densitometric analysis of myocardial blush grade10.
STATISTICAL METHODS
Results were presented as percentages of patients, medians with interquartile range, or mean and standard deviation, as applicable. Differences in categorical variables were analysed using a chi-square test and Fisher’s exact test. Continuous variables were compared using the Wilcoxon rank-sum test. The difference in mortality rates between groups during the one-year follow-up period was assessed by the Kaplan-Meier method, using the log-rank test. Multivariable models were constructed using the Cox proportional hazards model with stepwise selection. The following variables were considered for inclusion in the model: IRA TIMI 0 or 1 (vs. TIMI 2 or 3); age; gender; race; body mass index; Killip class on presentation; anaemia; platelet count; creatinine clearance <60; white cell count; left ventricular ejection fraction; assignment to bivalirudin (vs. UFH+GPI); stent type (DES vs. BMS); history of hypertension; hyperlipidaemia; smoking; diabetes; insulin treatment; prior myocardial infarction; coronary artery bypass graft surgery (CABG); angina; heart failure; peripheral vascular disease; clopidogrel loading dose; pre-randomisation UFH; baseline/discharge aspirin; baseline/discharge thienopyridines; symptom onset to first balloon inflation time; left anterior descending artery (LAD) disease; reference vessel diameter; the presence of thrombus, plaque ulceration, aneurysm, bifurcation lesion, and calcification; lesion length; and the number of stents used. All statistical tests were two-tailed and statistical significance was set at a level of 0.05, using SAS v9.2 (SAS Institute, Cary, NC, USA).
Results
BASELINE CHARACTERISTICS
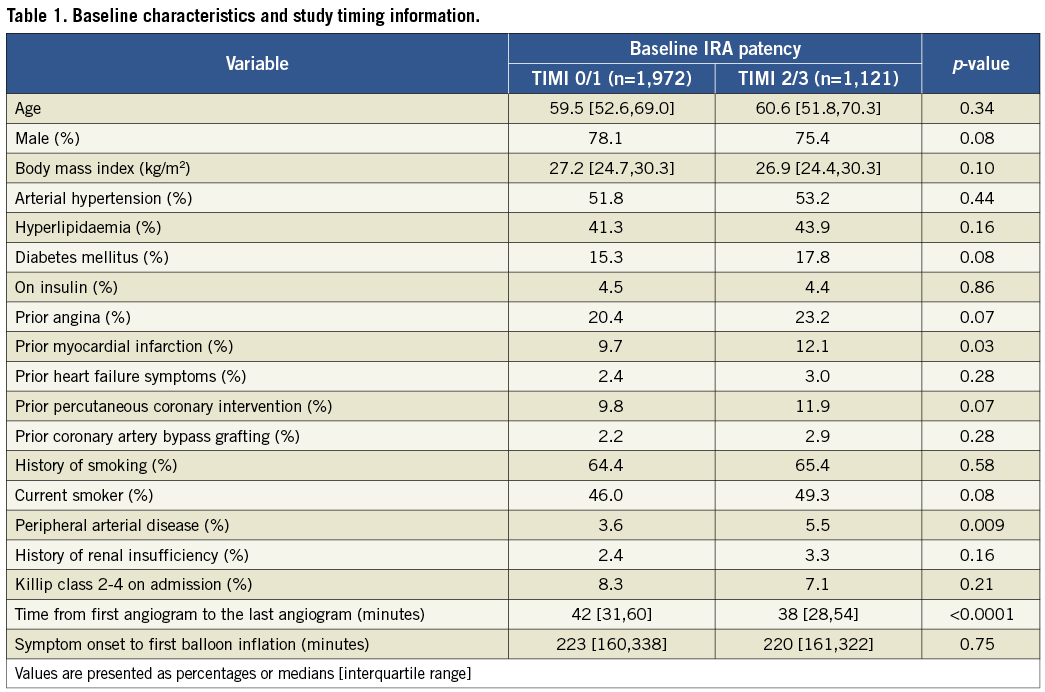
Of 3,602 enrolled patients, 3,093 (85.9%) were treated with primary PCI of a single IRA. Early IRA patency (TIMI 2 or 3 flow) was found in 1,121 patients (36.2%), whereas 1,972 patients (63.8%) had an occluded IRA (TIMI 0 or 1 flow). There were no significant differences in the baseline characteristics between the two groups except for a higher rate of prior myocardial infarction and peripheral artery disease in the patent IRA group (Table 1).
INTERVENTIONAL TREATMENT AND ELECTROCARDIOGRAPHIC RESULTS
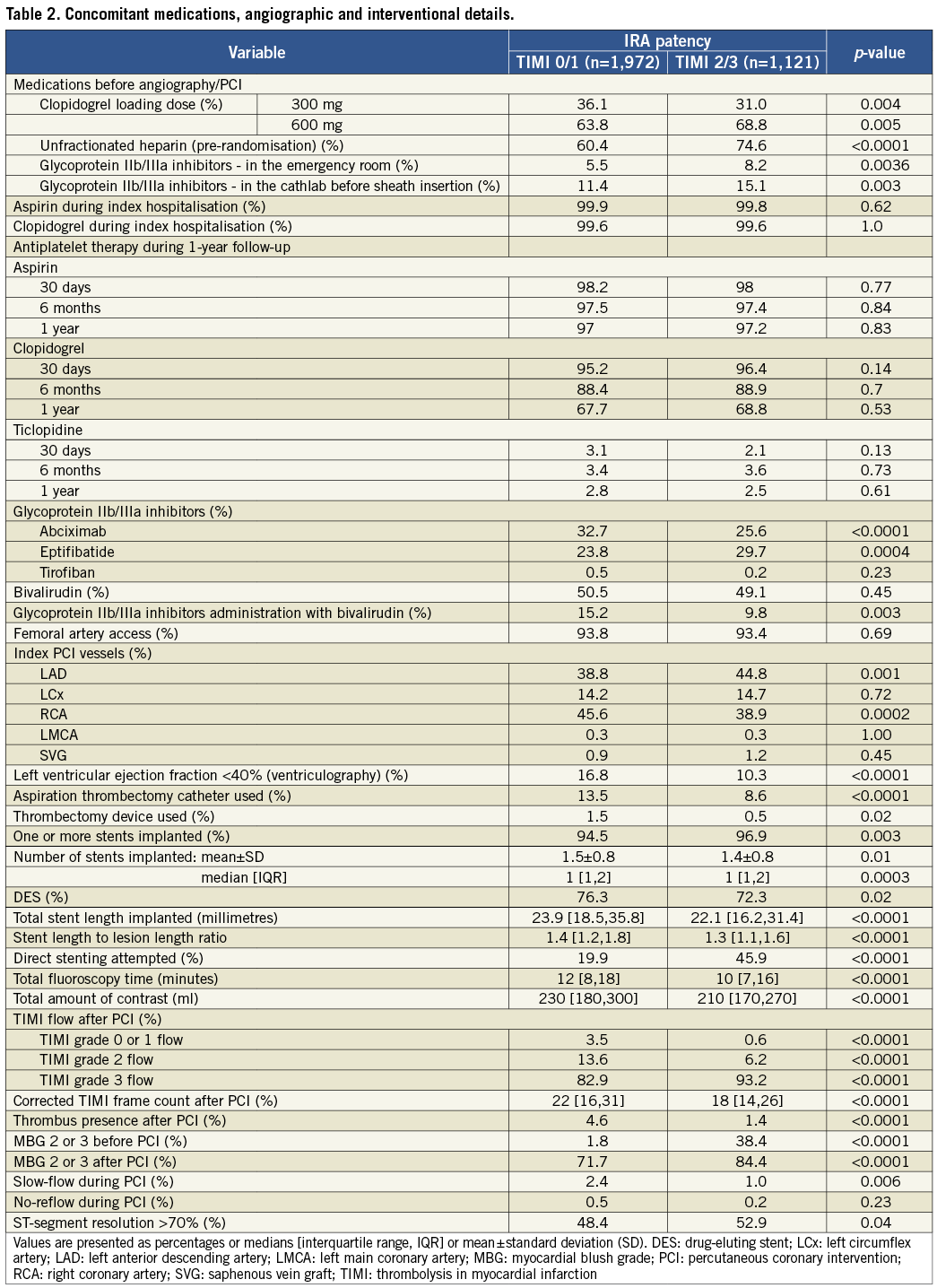
Total ischaemic time was similar in both groups. Procedure time was shorter in patients with early IRA patency (Table 1). Data concerning concomitant medications and interventional treatment are summarised in Table 2. Patients with patent IRA were more often treated with early administration of unfractionated heparin. Moreover, they tended to have received a clopidogrel loading dose of 600 mg instead of 300 mg. The rate of preprocedural administration of GPI was generally low but slightly higher in patients with patent IRA. There was no difference in aspirin and thienopyridine use during index hospitalisation between study groups. Bail-out treatment with GPI was more frequently used in the TIMI 0 or 1 group. Patients with, as compared to without, early IRA patency were more likely to have the LAD as the culprit artery, direct stenting, post-PCI TIMI 3 flow, post-PCI myocardial blush grade of 2 or 3, and ECG ST-segment resolution >70%.
CLINICAL OUTCOMES
The 30-day rates of all-cause death, MACE, NACE, and definite or probable stent thrombosis were significantly lower in patients with TIMI 2 or 3 flow at baseline angiography. The differences in mortality and stent thrombosis persisted at one-year follow-up. The difference in one-year stent thrombosis was mainly driven by the rate of acute and subacute stent thrombosis, and there was no significant difference in late stent thrombosis (Table 3). One-year event-free survival Kaplan-Meier curves for all-cause death are shown in Figure 2. The rate of major bleeding (non-CABG related) was not different between the two groups at 30 days or at one year (TIMI 0/1 vs. TIMI 2/3; 30 days: 7.7% vs. 6.1%, p=0.11; one year: 8.2% vs. 6.6%, p=0.12).
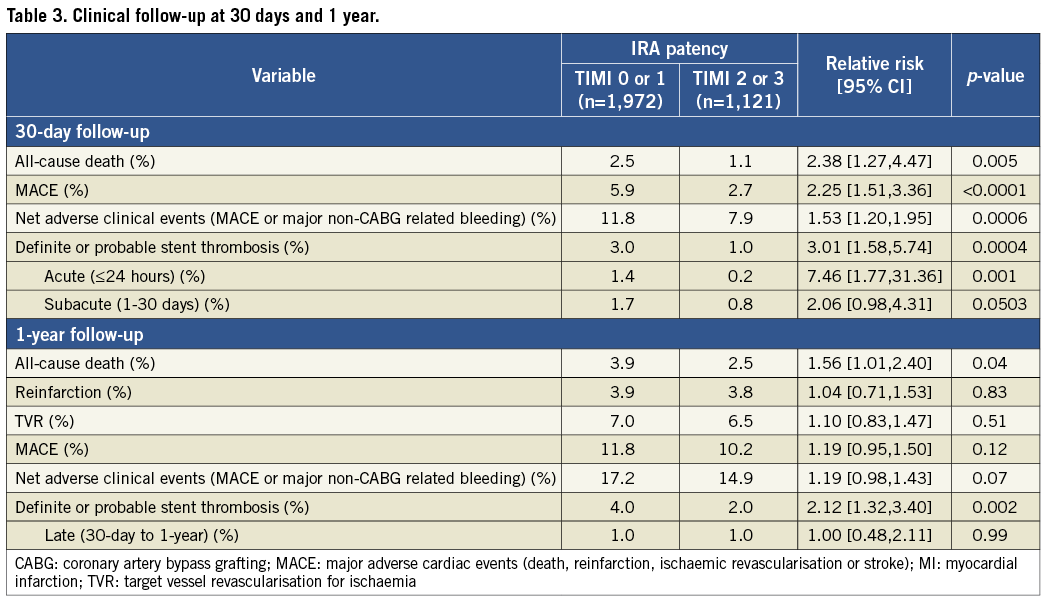
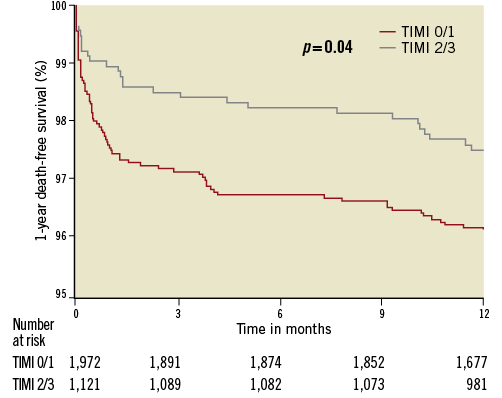
Figure 2. Kaplan-Meier curves for 1-year death-free survival according to baseline infarct-related artery patency (TIMI flow 0/1 vs. TIMI flow 2/3).
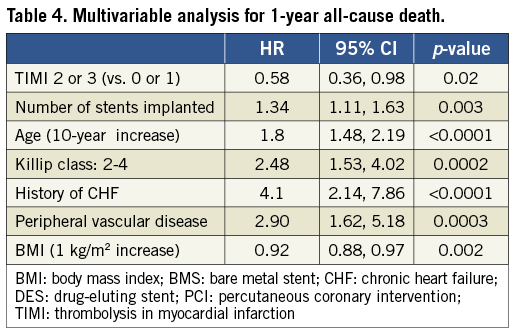
MULTIVARIABLE ANALYSIS
In multivariable analysis, patent IRA at baseline angiography was an independent predictor of lower mortality at one year (Table 4).
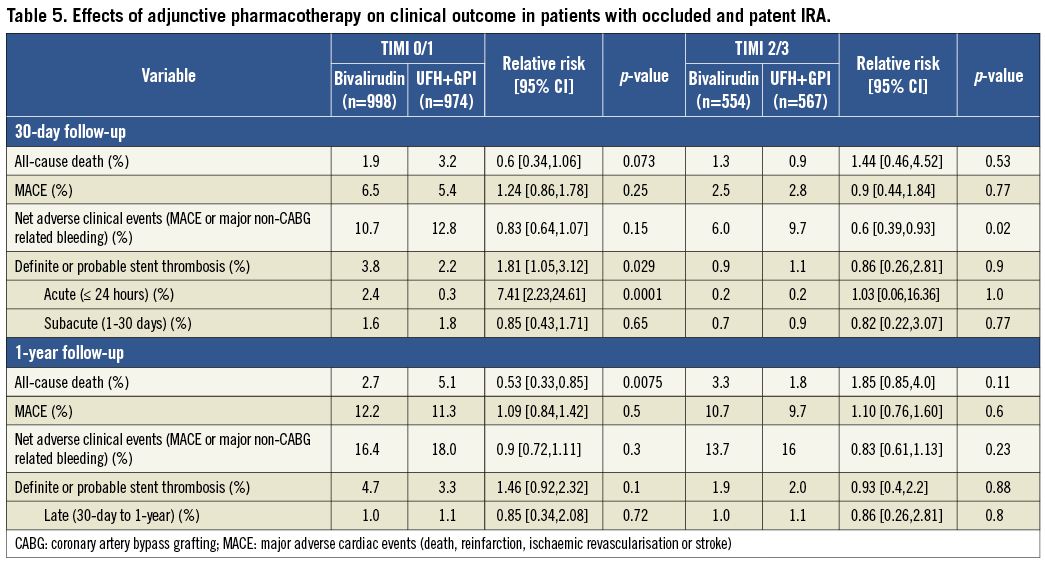
EFFECTS OF ADJUNCTIVE PHARMACOTHERAPY ON CLINICAL OUTCOME IN PATIENTS WITH OCCLUDED AND PATENT IRA
The definite or probable stent thrombosis rate was similar after bivalirudin and UFH plus GPI treatment both for 30-day and one-year follow-up in patients with patent IRA. However, a higher rate of acute stent thrombosis was found with bivalirudin compared to UFH plus GPI in patients with occluded IRA at baseline. During one-year follow-up there were no significant differences in event rates after bivalirudin compared to UFH plus GPI for both occluded and patent IRA, other than lower mortality at one-year after bivalirudin in patients with occluded IRA (Table 5).
Discussion
In the HORIZONS-AMI trial, early IRA patency was independently associated with better procedural results and improved one-year clinical outcomes.
Acute ischaemia caused by occlusion of the IRA leads to myocardial necrosis within hours. Despite many factors affecting the progression of myocardial injury, duration of ischaemia remains the most important determinant of infarct size11. Longer time to reperfusion in patients with STEMI is associated with worse long-term clinical outcome regardless of the type of reperfusion strategy used. Available data confirm the superiority of primary PCI over fibrinolysis for patients with STEMI, but delay to mechanical reperfusion may reduce the benefit of this approach12,13. In some patients with STEMI, spontaneous reperfusion occurs before angiography and PCI. Stone et al analysed the four Primary Angioplasty in Myocardial Infarction (PAMI) trials and showed that normal flow (TIMI 3) before primary PCI is an independent determinant of survival in acute myocardial infarction3. Similar observations were presented by Brodie et al for the comparison of TIMI flow 0/1 vs. 2/3, and by De Luca et al for TIMI 3 vs. non-TIMI 3 flow. Both analyses were based on registry data collected before 20004,5. The benefit of early IRA recanalisation was more pronounced in high-risk patients5. Based on the importance of time delay to primary PCI and the beneficial effect of early reperfusion, the concept of pharmacologically induced reperfusion was born. Theoretical advantages of early IRA patency include easier guidewire passage, better stent selection, and smaller thrombus burden with lower risk of distal embolisation. This strategy has been tested in many clinical trials using thrombolytics, GPIs or a combination of both, but has not shown clinical benefit over primary PCI14, primarily because of increased bleeding. Since that time, there has been a multitude of changes in various aspects of primary PCI, ranging from changes in adjunctive pharmacotherapy such as bivalirudin and clopidogrel, to improvements in stent design and drug elution and aspiration thrombectomy, that have resulted in improved outcomes in both the short term and the long term. Thus, this analysis updates historical data with a more contemporary cohort. Besides the difference in mortality rate previously described, we have observed that patients with occluded IRA at baseline angiography are at higher risk of acute and subacute stent thrombosis compared to patients with patent IRA at baseline. The risk of acute stent thrombosis was especially pronounced in patients with occluded IRA treated with bivalirudin. This highlights the importance of more aggressive antiplatelet and antithrombotic treatments in patients with large thrombus burden and occluded IRA. Further studies are needed to determine whether the risk of acute and subacute stent thrombosis in such patients may be reduced by more potent and rapidly acting thienopyridines such as prasugrel or ticagrelor, or by prolonged bivalirudin infusion15,16. Thrombus burden was previously described as an independent predictor of stent thrombosis in STEMI patients treated with DES stent implantation17. New devices including aspiration thrombectomy and specially designed stents (e.g., mesh cover stents) may be beneficial in patients with large thrombus burden18-20.
Lack of compliance to dual antiplatelet therapy is an important risk factor for stent thrombosis21. However, at 30-day follow-up, when a significant difference in the stent thrombosis rate between the two patency groups was observed, the adherence to aspirin and thienopyridine treatment was over 98% in both groups.
Our data warrant future studies to promote early reperfusion prior to primary PCI. This may include early administration of new oral antiplatelet drugs (prasugrel, ticagrelor) or new anticoagulants like bivalirudin. Such an approach is evaluated in the ATLANTIC Trial on STEMI patients treated with early vs. late ticagrelor administration and with IRA patency as a primary endpoint. The role of early prasugrel administration is analysed in the ongoing ACCOAST Trial in patients with non-ST-segment elevation myocardial infarction. Furthermore, in the EUROMAX Trial a strategy of early bivalirudin administration is currently being evaluated.
Limitations
The present study has a number of limitations. Data were analysed post hoc and the randomisation was not stratified according to baseline IRA patency. However, study groups were well matched in terms of baseline characteristics and we used multivariable analysis to limit the influence of confounders. We have taken one-year instead of longer follow-up data for clinical outcome analysis, in order to remove the effect of the increase in event rates following angiographic follow-up scheduled for 13 months22. Aspiration thrombectomy devices were used infrequently in the study. Our results may not be applied to patients excluded from randomisation. Moreover, for the purpose of the analysis, we have included only patients treated with single-vessel PCI, so the results may not be extrapolated to patients treated with deferred PCI, multivessel PCI, CABG or medical management who were excluded.
Conclusions
Early IRA patency at baseline angiography in patients with STEMI undergoing primary PCI is associated with better revascularisation and improved one-year clinical outcomes including lower rate of death and stent thrombosis. These data warrant further studies to promote early reperfusion prior to primary PCI.
Conflict of interest statement
D. Dudek received grant support from: Boston Scientific Corporation, St. Jude Medical, Volcano Corporation, and is a consultant to Boston Scientific Corporation, St. Jude Medical. B. Witzenbichler received lecture fees from Boston Scientific and The Medicines Company. G. Guagliumi received grant support from: Abbott Vascular, Boston Scientific Corporation, St. Jude Medical, and is a consultant to: Boston Scientific Corporation, St. Jude Medical. R. Mehran received Institutional Research Grant Support from: The Medicines Company, Bristol-Myers Squibb/Sanofi, Eli Lilly and Company/Daiichi-Sankyo, and is a consultant to: Abbott Vascular, AstraZeneca, Janssen Pharmaceuticals, Regado Biosciences, The Medicines Company, Bristol-Myers Squibb/Sanofi, and Merck & Co. G.W. Stone is a consultant to Boston Scientific Corporation. T. Rakowski, A. Dziewierz, J. Yu, R. Kornowski, F. Hartmann, A.J. Lansky and S.J. Brener have no conflicts of interest to declare.

