Abstract
Aims: The predictors of TIMI flow <3 after PCI in patients with acute myocardial infarction have not been examined in a contemporary, large-scale multicentre prospective study.
Methods and results: The HORIZONS-AMI trial randomised 3,602 patients with STEMI undergoing primary PCI to bivalirudin (n=1,800) vs. unfractionated heparin plus a glycoprotein IIb/IIIa inhibitor (n=1,802). A total of 3,845 treated lesions (3,362 vessels) were analysed by the core lab; 2,942 vessels (87.5%) and 2,758 patients (87.1%) had final TIMI 3 flow, while 407 (12.9%) had TIMI flow <3. The independent predictors of TIMI flow <3 were age (OR 1.23 per 10-year increase; 95% CI: 1.12 to 1.35; p<0.0001), anterior MI (OR 1.65; 95% CI: 1.33 to 2.05; p<0.0001), baseline TIMI flow grade 0/1 (OR 2.79; 95% CI: 2.14 to 3.62; p<0.0001), and lesion length (OR 1.05 per 10 mm increase; 95% CI: 1.02 to 1.09; p=0.005). The three-year mortality of patients in whom final TIMI 3 flow was achieved was significantly lower than that of patients in whom TIMI 3 flow was not achieved (5.5% vs. 10.5%; p<0.0001).
Conclusions: In this large-scale, randomised trial, failure to restore normal TIMI flow after primary PCI in STEMI occurred in 12.9% of patients, and was associated with patient-related factors (age), anatomical factors (anterior MI location), and angiographic factors (baseline TIMI 0/1 flow and lesion length). Failure to achieve TIMI 3 flow continues to be a powerful predictor of mortality after primary PCI in the contemporary era. Clinical Trial Registration: http://www.clinicaltrials.gov. Unique identifier: NCT00433966.
Introduction
Primary percutaneous coronary intervention (PCI) improves survival rates in patients with acute myocardial infarction (MI) as compared with thrombolytic therapy, in large part due to better and sustained antegrade epicardial perfusion (thrombolysis in myocardial infarction [TIMI] grade 3 flow)1. As compared with balloon angioplasty, primary PCI with stent implantation results in a significant and persistent reduction in recurrent ischaemia and target vessel revascularisation2,3. Hence, current guidelines recommend primary PCI with stent implantation as the treatment of choice for patients with evolving ST-segment elevation myocardial infarction (STEMI)4. Nevertheless, failure to achieve TIMI 3 flow (i.e., suboptimal TIMI flow) after primary PCI still occurs in up to 5-23%2,3,5,6 of patients. Prior studies have reported a strong relationship between suboptimal final TIMI flow and mortality5,7,8. However, the impact of suboptimal TIMI flow on long-term follow-up (>1 year) has never previously been reported. The Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI)9 trial provides a unique opportunity to investigate the long-term prognostic utility and baseline clinical and angiographic predictors of suboptimal TIMI flow after primary PCI in the contemporary era.
Methods
PATIENT POPULATION AND STUDY PROTOCOL
The HORIZONS-AMI trial design has been described elsewhere in detail10. In brief, HORIZONS-AMI was a prospective, randomised, multicentre trial of 3,602 STEMI patients undergoing primary PCI. Patients were randomised prior to angiography in a 1:1 ratio to either unfractionated heparin (UFH) plus routine use of a glycoprotein IIb/IIIa inhibitor (GPI) or bivalirudin monotherapy. Following angiography, 3,006 eligible patients underwent a second randomisation (3:1) to either TAXUSTM Express2TM paclitaxel-eluting stents (PES) (Boston Scientific, Natick, MA, USA) or uncoated Express2TM bare metal stents (BMS) (Boston Scientific). The decision whether to use a manual thrombus aspiration device before or during PCI was left to the operator’s discretion. A clopidogrel loading dose (either 300 mg or 600 mg) was administered prior to catheterisation, followed by 75 mg PO daily for at least six months (one year recommended).
STUDY OBJECTIVES AND DEFINITIONS
The primary objective of this study was to evaluate the angiographic, clinical and procedural predictors of core-laboratory assessed suboptimal final TIMI flow (<3) after stent implantation. A secondary objective was to assess the impact of final suboptimal TIMI flow (TIMI 0-2 vs. TIMI 3) on three-year clinical outcomes. All adverse events were adjudicated by an independent clinical events committee, blinded to pharmacology and stent assignment, after review of original source documentation.
ANGIOGRAPHIC ANALYSIS
An independent angiographic laboratory performed quantitative coronary angiography on all baseline and post-PCI lesions (CMS; Medis Medical Imaging Systems, Leiden, The Netherlands), blinded to randomisation assignment and clinical outcomes (Cardiovascular Research Foundation, New York, NY, USA). TIMI flow in the infarct vessel was assessed as previously reported11.
STATISTICAL ANALYSIS
Categorical variables were compared by chi-square analysis or Fisher’s exact test. Continuous variables are presented as mean ± SD, median and interquartile ranges (IQR) and were compared by Student’s t-test or Wilcoxon rank-sum test, as appropriate. Kaplan-Meier methods were used to derive the event rates at follow-up and to plot time-to-event curves; comparisons were made using the log-rank test. The Cox proportional hazards model was used to derive the independent predictors of death and cardiac death, and stepwise logistic regression was used to determine the independent predictors of final suboptimal TIMI flow (<3). Lesion level variables were summarised as worst TIMI flow per patient if multiple lesions were present. The p-value to enter and exit each model was 0.2/0.1. All statistical tests were two-tailed. Statistical significance was set at a level of 0.05. All analyses were performed with SAS 9.0 (Cary, NC, USA).
Results
FREQUENCY AND CORRELATES OF SUBOPTIMAL FINAL TIMI FLOW
PCI was performed in 3,345 patients (92.9%), among whom stents were implanted in 3,226 (96.4%). A total of 3,845 stented lesions (3,362 vessels in 3,165 patients) were analysed by the core lab and comprise the current study cohort; 2,758 patients (87.1%) had final TIMI 3 flow, and 407 (12.9%) had TIMI flow <3. Compared to patients with final TIMI 3 flow, patients with final TIMI flow <3 were more likely to be older, have more renal insufficiency, more evidence of heart failure (Killip class 2-4) and left ventricular dysfunction at presentation, and were less likely to be smokers or to have prior MI or PCI. Additionally, patients with final TIMI flow <3 had significantly longer times from symptom onset to first hospital arrival, from symptom onset to balloon, and from door to balloon (Table 1).
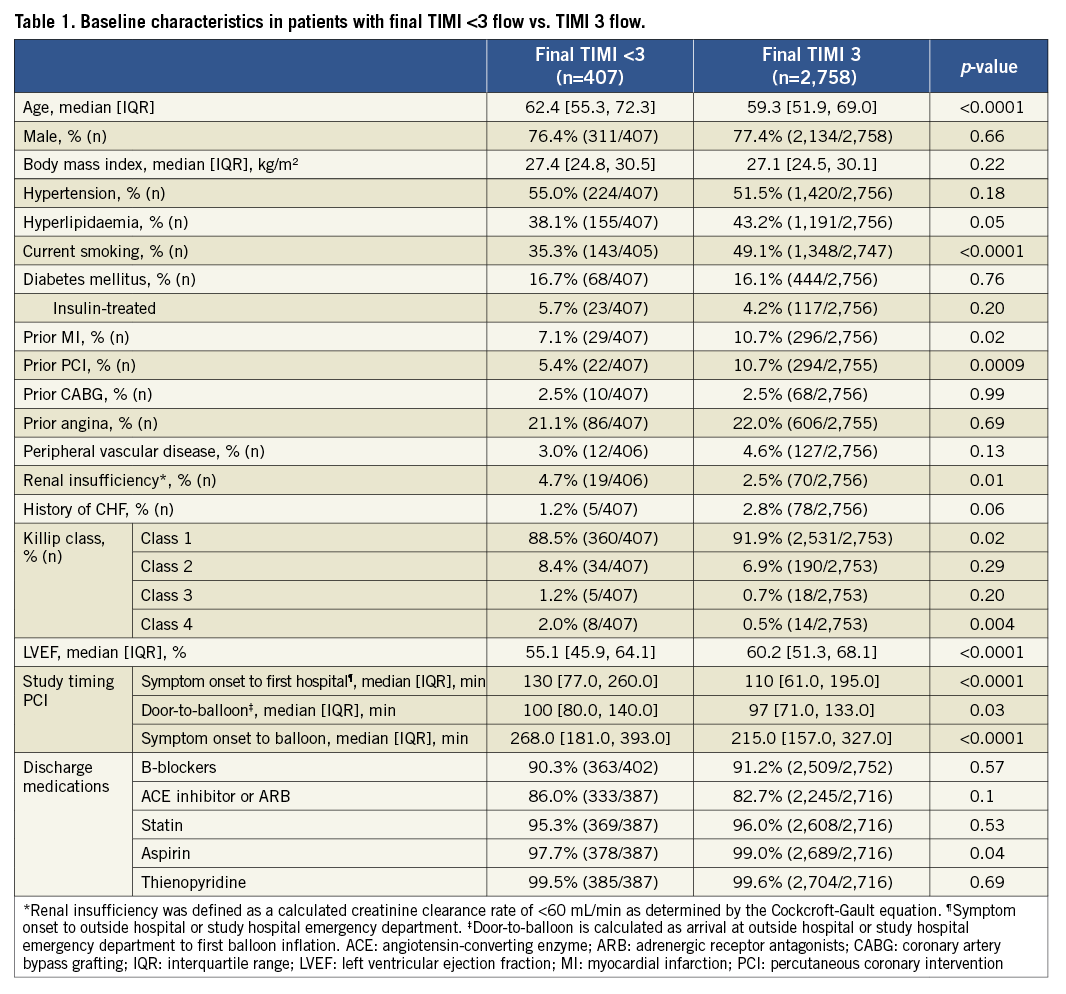
Lesions in patients with final TIMI flow <3 were more likely to be located in the proximal and mid left anterior descending artery, had a larger baseline thrombus burden, were more complex (by ACC/AHA classification), and had lower epicardial TIMI flow before the procedure (Table 2). An aspiration catheter was used more frequently in patients with final TIMI flow <3 compared with those with TIMI 3 flow (14.8% vs. 10.9%; p=0.02). TIMI 3 flow was restored in a comparable percentage of patients randomised to bivalirudin and UFH plus GPI (86.6% vs. 88.3%, respectively, p=0.15).
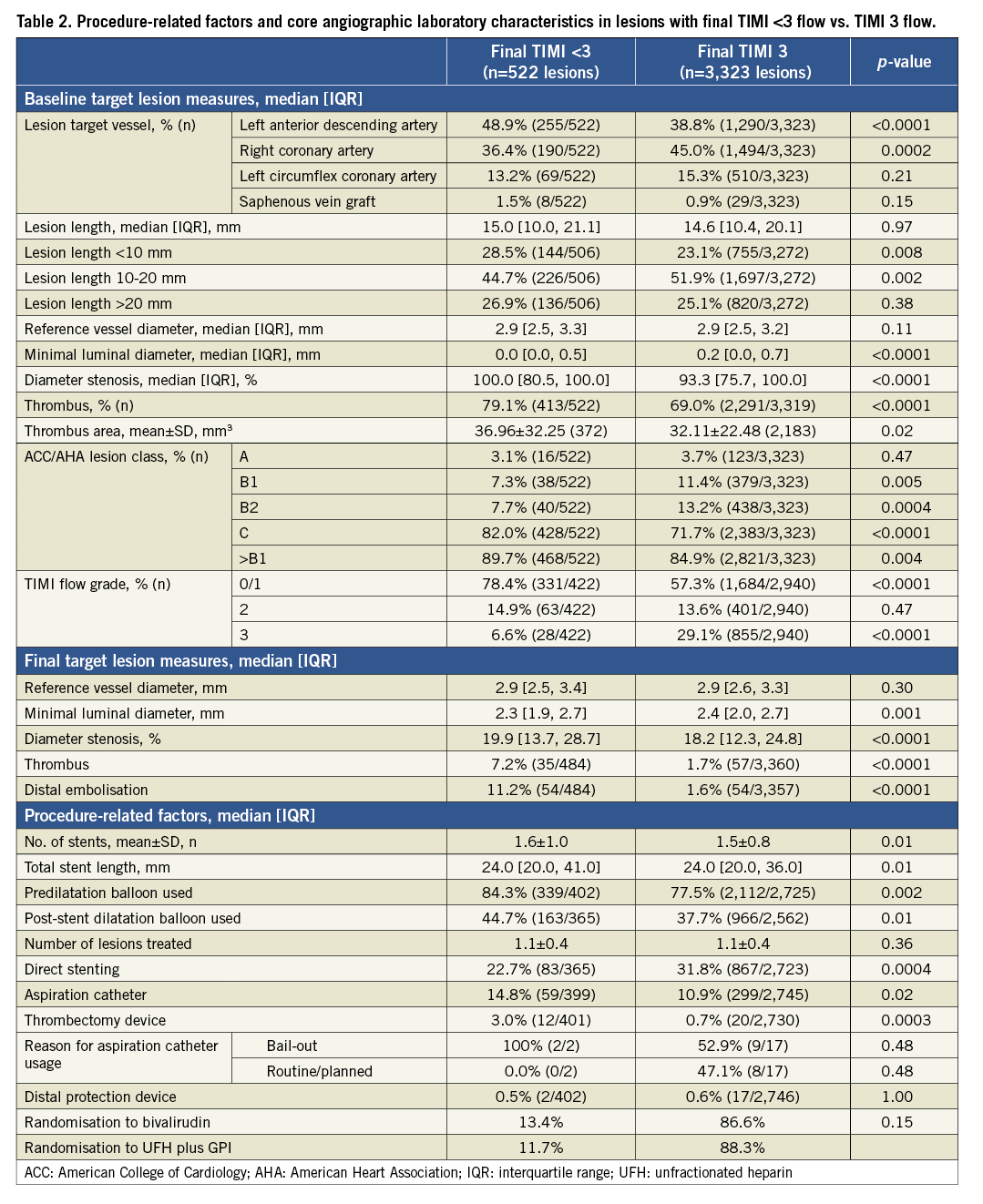
PREDICTORS OF SUBOPTIMAL TIMI FLOW
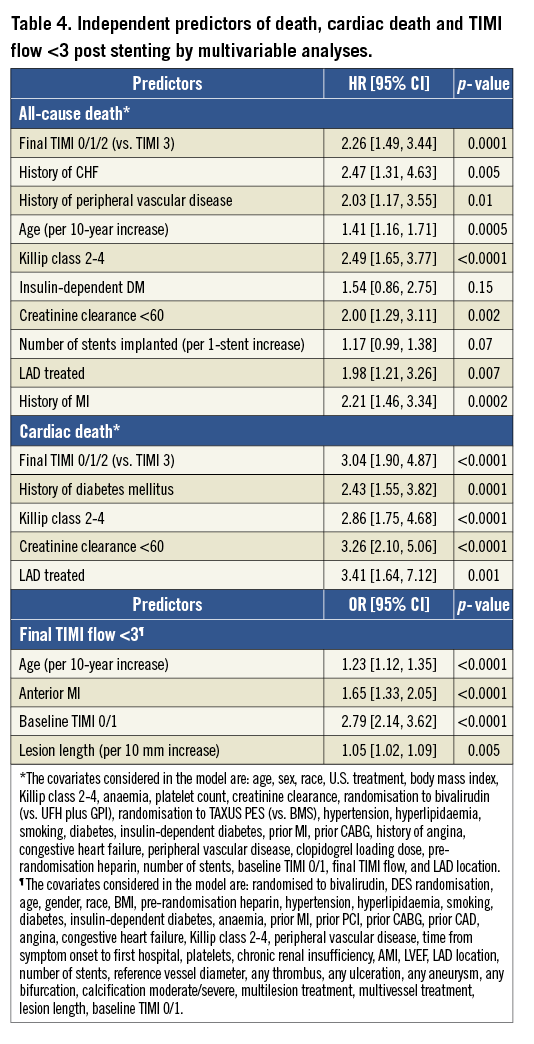
By multivariable analysis, the independent predictors of TIMI flow <3 were age (OR 1.23 per 10-year increase; 95% CI: 1.12 to 1.35; p<0.0001), anterior MI (OR 1.65; 95% CI: 1.33 to 2.05; p<0.0001), baseline TIMI flow grade 0/1 (OR 2.79; 95% CI: 2.14 to 3.62; p<0.0001), and lesion length (OR 1.05 per 10 mm increase; 95% CI: 1.02 to 1.09; p=0.005).
IMPACT OF FINAL TIMI FLOW ON CLINICAL OUTCOMES
The 30-day and three-year rates of major adverse cardiovascular events (MACE) and bleeding were significantly higher in patients with TIMI flow <3 compared to those with TIMI 3 flow (Table 3 and Figure 1). After multivariable adjustment for differences in baseline covariates, final TIMI flow <3 independently predicted all-cause death and cardiac death at three years (Table 4). STEMI patients with final TIMI flow <3 compared with those with TIMI 3 flow had an adjusted HR for all-cause mortality of 2.26 (95% CI: 1.49 to 3.44; p=0.0001) and an adjusted HR for cardiac mortality of 3.04 (95% CI: 1.90 to 3.44; p=0.0001) at three years. Other independent predictors of three-year cardiac mortality in addition to TIMI flow <3 were a history of diabetes, Killip class 2-4, creatinine clearance <60 ml/min and treatment in the left anterior descending artery.
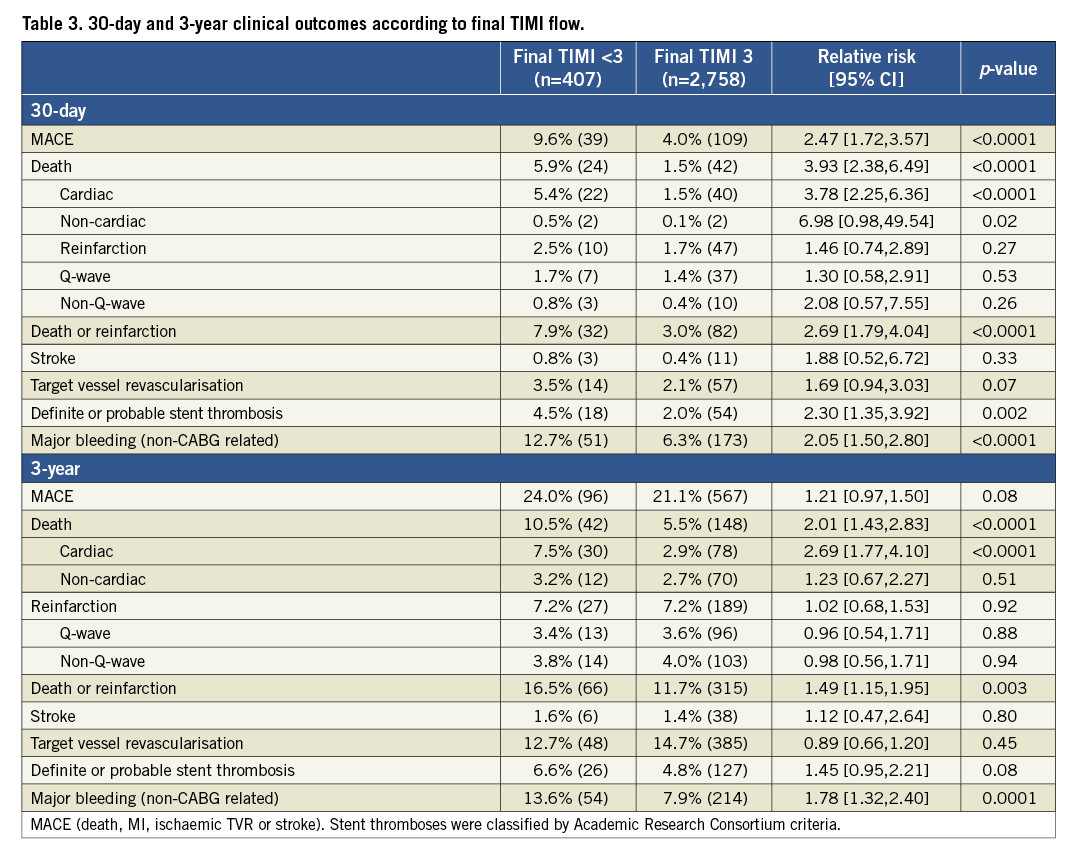
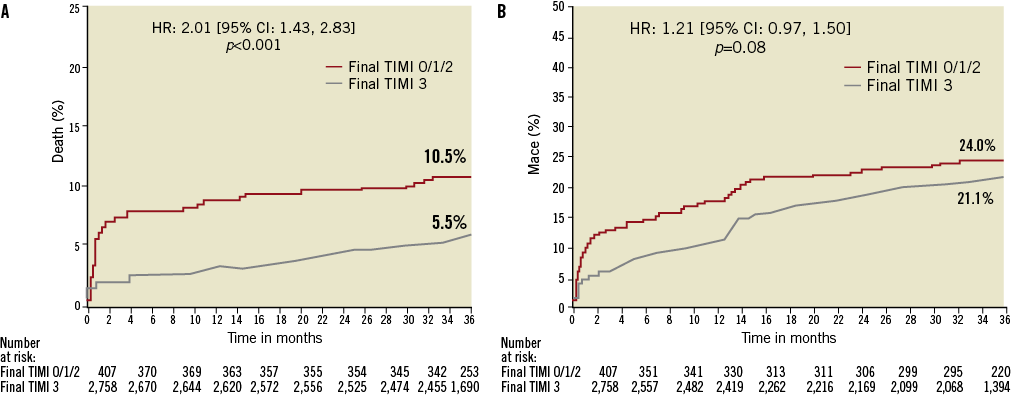
Figure 1. Time-to-events curves for patients with final suboptimal TIMI flow (TIMI 0-2) vs. TIMI 3 flow. Three-year cumulative event curves for (A) all-cause mortality, and (B) MACE.
Discussion
The present analysis from the large-scale, multicentre, prospective randomised HORIZONS-AMI trial demonstrates that failure to achieve normal TIMI flow after primary PCI in STEMI is relatively common (12.9%), and is associated with patient-related factors (age), anatomical factors (anterior MI location) and angiographic factors (baseline TIMI 0/1 flow and lesion length). In addition, not achieving TIMI 3 flow continues to be a powerful predictor of short and long-term mortality in the contemporary primary PCI era.
SUBOPTIMAL TIMI FLOW AND IMPACT ON MORTALITY
The findings of the present study are in agreement with previous reports showing the relationship between final suboptimal epicardial TIMI flow after PCI and mortality in STEMI patients5,7,8,12,13. In a pooled analysis from the four major Primary Angioplasty in Myocardial Infarction (PAMI) trials7, patients with post-procedure TIMI flow <3 (7.5%) had a fourfold increased risk of six-month mortality. Likewise, patients with suboptimal TIMI flow (5%) after stent implantation experienced a significant threefold increased risk of death at six months in the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial8. Finally, in the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial5 patients with TIMI flow <3 (15%) had threefold higher mortality at six months. The present study extends these findings by demonstrating the prognostic value of final TIMI flow <3 in a larger population with fewer exclusion criteria than from earlier studies, treated with contemporary pharmacotherapy. While the PAMI trials7 and APEX-AMI5 included patients within six hours of symptom onset, and the CADILLAC trial8 selected patients with angiographically less complex anatomy, HORIZONS-AMI enrolment criteria permitted a broader clinical presentation (within 12 hours of symptom onset) and more complex coronary anatomy and angiographic lesion subsets. Moreover, the present analysis represents the longest follow-up (three years) thus far describing the impact of epicardial TIMI flow grade at the index PCI on very late mortality. Of note, suboptimal post-PCI TIMI flow was an independent predictor of mortality even after correction for baseline TIMI flow; and a three-year higher cumulative rate of mortality in patients with suboptimal TIMI flow was driven by events within the initial two months. The survival benefit of PCI over thrombolytic therapy is in large part due to the achievement of higher rates of epicardial TIMI 3 blood flow1. The pathophysiology of slow flow and no-reflow after primary PCI is multifactorial14. Despite TIMI flow grade interpretation being subject to interobserver variability15, it is a simple, quick and reliable evaluation that strongly predicts long-term mortality (although other surrogate markers of impaired myocardial perfusion have higher sensitivity than TIMI flow grade for the prediction of death, including myocardial blush grade, ST-segment resolution and TIMI frame count)5,16,17.
MODIFIABLE PREDICTORS OF FINAL TIMI FLOW
In our study, baseline TIMI 0/1 flow was a powerful predictor of suboptimal final TIMI 3 flow. Similarly, in the APEX-AMI trial5 primary PCI study, the independent predictors of achieving suboptimal TIMI flow after PCI were age, time to treatment and pre-PCI TIMI flow grade 0/1. Ndrepepa et al13 reported that abnormal baseline TIMI flow is an independent predictor of no-reflow after PCI. Stone et al7 found preprocedural TIMI <3 flow was an independent predictor of mortality in patients with STEMI. Nevertheless, the lack of success of facilitated PCI18-20 in improving final coronary flow with half dose6 and full dose21 thrombolytic agents or improving prognosis after STEMI highlights the inherent differences between the spontaneous and pharmacologic-induced restoration of epicardial perfusion prior to PCI. Conversely, use of adjunctive manual thrombectomy in STEMI patients has been associated with significant improvement in coronary flow before stent implantation as well as postprocedural epicardial and myocardial perfusion with less distal embolisation, and possible benefits in long-term survival22. While a large-scale randomised trial is ongoing to determine the ability of aspiration thrombectomy to reduce mortality in STEMI (Thrombus Aspiration in Myocardial Infarction [TASTE], NCT01093404), the present data from the HORIZONS-AMI trial support this hypothesis. The HORIZONS-AMI trial was designed before the routine incorporation of manual thrombus aspiration in the setting of STEMI and this technique was used in only 10% of the patients. Furthermore, the higher usage of thrombus aspiration catheters or any thrombectomy device in patients with suboptimal TIMI flow was driven by unplanned indication (e.g., bailout or to treat suboptimal results). Thus, the impact of these devices in mitigating suboptimal final TIMI flow in the present analysis could not be properly assessed. Finally, there is also an ongoing debate regarding the use of superselective intracoronary administration of abciximab in STEMI patients. Intracoronary infusion of abciximab significantly reduced thrombus volume with improvement in final TIMI flow23, as well as resulting in a significant reduction in infarct size in the recent Intracoronary Abciximab and Aspiration Thrombectomy in Patients with Large Anterior Myocardial Infarction (INFUSE-AMI) trial24. Larger studies will be needed to determine whether the degree of reduction in infarct size and improvement in TIMI flow obtained with the intracoronary infusion of abciximab translates into improved clinical outcomes.
In the present analysis, there was no interaction between time to treatment (symptom onset to balloon dilatation) and either suboptimal final TIMI flow or mortality. Although the mortality benefit of thrombolytic therapy for acute MI is strongly dependent on time to treatment1, its role in patients undergoing primary PCI remains controversial; some studies suggest that time delay to treatment increases mortality5, whereas others report no effect25. The lack of correlation between time to treatment and suboptimal TIMI flow and mortality in the present analysis suggests that the survival benefit from primary PCI may be influenced by factors other than myocardial salvage (e.g., major bleeding, transfusion, and thrombocytopenia). Although the exact mechanism is unclear, it is possible that an association between time to treatment and suboptimal TIMI flow has been overridden by other powerful covariates such as baseline TIMI flow. However, the finding that anterior MI was an independent correlate of suboptimal final TIMI flow corroborates previous studies that have demonstrated a close correlation between infarct size, MI involving the LAD distribution, and the presence of abnormal TIMI flow13,26. LAD infarctions have a higher initial area at risk13,27, cause more severe systolic dysfunction prior to PCI, and are more likely to result in distal embolisation after PCI, a potential mechanism of slow flow and no-reflow.
Limitations
The present data represent the largest QCA database pertaining to STEMI patients; nevertheless, we cannot completely rule out the possibility that not all multivariable correlates of suboptimal TIMI flow were identified. Finally, we did not assess the association between final TIMI flow and other angiographic surrogate markers of myocardial perfusion (e.g., myocardial blush and TIMI frame count); we therefore could not investigate their relative risk prediction of clinical outcomes.
Conclusions
In the large-scale, multicentre, prospective, randomised HORIZONS-AMI trial, absence of normal TIMI 3 flow after primary PCI in STEMI occurred in 12.9% of patients, and was associated with patient-related factors (age), anatomical factors (anterior MI location), and angiographic factors (baseline TIMI 0/1 flow and lesion length). Failure to achieve TIMI 3 flow continues to be a powerful predictor of mortality after primary PCI in the contemporary reperfusion era.
Funding
HORIZONS-AMI was supported by the Cardiovascular Research Foundation, with unrestricted grant support from Boston Scientific Corporation and The Medicines Company.
Conflict of interest statement
R. Mehran received a research grant from Sanofi-Aventis, honoraria from The Medicines Company, Abbott Vascular, Sanofi-Aventis, Bristol-Myers Squibb, Astra-Zeneca and Cordis. She is a consultant or on the advisory board of Eli Lilly and Daiichi Sankyo. B. Witzenbichler received honoraria from Boston Scientific and The Medicines Company. G. Guagliumi received research grants from Medtronic, Boston Scientific, LightLab Imaging and Abbott Vascular and is a consultant and/or on the advisory board of Boston Scientific, Volcano and Cordis. D. Dudek received honoraria from Nycomed. M. Fahy is an employee of the Cardiovascular Research Foundation. B. Brodie received honoraria from The Medicines Company and MedRad/Possis. G. Dangas received honoraria from The Medicines Company, Sanofi-Aventis, Bristol-Myers Squibb, Astra-Zeneca, Cordis and Abbott Vascular. He is a consultant and/or on the advisory board of Eli Lilly and Daiichi Sankyo. G. Stone is a consultant and/or on advisory board of The Medicines Company, Abbott Vascular, Boston Scientific and Medtronic. The other authors have no conflicts of interest to declare.

