
Abstract
Aims: Our aim was to compare stenosis severity and plaque content between STEMI culprit lesions with intact fibrous cap (IFC) and those with plaque rupture (PR) in a prospective study.
Methods and results: We evaluated 93 patients undergoing OCT and thrombectomy as part of a prospective substudy of the TOTAL (ThrOmbecTomy versus PCI ALone) trial. Culprit lesion morphology was assessable by OCT in 70/93 (75.3%). IFC was found in 31 (44.3%), PR in 34 (48.6%) and calcified nodule in five (7.1%) patients. Following thrombectomy, OCT demonstrated similar lumen area stenosis in IFC (79.3%) and PR (79.6%) (p=0.88). Lumen area stenosis <50% was observed in none of the patients with PR and in one patient with IFC. IFC had fewer quadrants with lipid plaque as compared to PR (28.16±15.02 vs. 39.12±14.23, p=0.004). However, in both lesion types, lipid was the predominant plaque type (83.9 vs. 63.7% of diseased quadrants).
Conclusions: In a prospective study of STEMI patients treated with thrombectomy, mild residual stenoses were uncommon in IFC lesions. Although lipid content was lower than in PR lesions, lipid composed the majority of the diseased segments in IFC.
Introduction
The most frequent culprit lesion morphologies of ST-segment elevation myocardial infarction (STEMI) identified by optical coherence tomography (OCT) are plaque rupture (PR) and intact fibrous cap (IFC)1,2. Calcified nodules are observed in a minority of cases3. Recently, several studies have reported a considerable (26.8-39%) frequency of IFC in STEMI patients4-7.
Prior data have suggested that some patients with STEMI with IFC may have mild stenoses after thrombectomy8, which may be treated without stent implantation. In addition, the plaque morphology of IFC culprit lesions has not been sufficiently clarified. In previous studies, IFC culprit lesions were found to have less lipid-rich plaque and/or less frequent thin-cap fibroatheroma4-7. A recent near-infrared spectroscopy (NIRS) study, however, showed high lipid content in almost all STEMI culprit lesions, although it was not possible for this technique to evaluate PR9. Therefore, the true lipid content of IFC lesions is unclear.
We conducted a prospective study to evaluate residual stenosis severity and plaque morphology by lesion type in patients undergoing thrombectomy in the multicentre TOTAL-OCT study.
Methods
STUDY DESIGN AND PATIENTS
This report includes patients enrolled in the OCT substudy of the TOTAL (ThrOmbecTomy versus PCI ALone) trial. Patients of the OCT substudy (N=214) were enrolled at 13 centres, as previously described10,11. For this analysis, we included patients who had culprit lesion imaging without balloon predilataton. The TOTAL trial was an international, multicentre, randomised trial of routine thrombectomy (using the Export family of catheters; Medtronic Cardiovascular, Santa Rosa, CA, USA) compared with PCI alone in STEMI patients treated with primary PCI (N=10,732)12. Enrolled patients presented with symptoms of myocardial ischaemia lasting for ≥30 minutes and definite electrocardiographic changes indicating STEMI who were referred for primary PCI and randomised within 12 hours of symptom onset13. In addition to the exclusion criteria of the main TOTAL trial, patients with cardiogenic shock or known renal failure were excluded from the OCT substudy. The study was approved by the local human research ethics committees of the recruiting hospitals. Informed consent specifically for the substudy was provided by all patients enrolled.
OCT IMAGING
OCT imaging was performed after the trial-mandated thrombectomy using the ILUMIEN™ OCT system and C7 Dragonfly™ catheter (St. Jude Medical, St. Paul, MN, USA). The radiopaque distal marker of the OCT catheter was positioned 1-2 cm distal to the target lesion or stent and contrast was injected either manually or by automatic injector, depending on local practice. Adequate image quality was checked and additional images were obtained, if the image quality was suboptimal. A maximum of three attempts was allowed. OCT imaging raw data were exported in digital format for off-line analysis.
ANGIOGRAM ANALYSIS
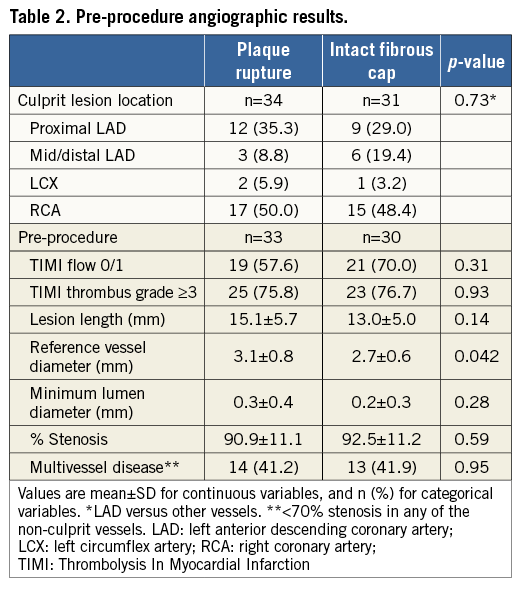
A dedicated angiographic core laboratory (Hamilton Health Sciences, Hamilton, ON, Canada) assessed angiograms of patients enrolled in the OCT substudy. Readers were blinded to the clinical data and OCT findings. Pre-stent angiographic TIMI thrombus grade was assessed at the first injection of the infarct-related artery13, and quantitative coronary angiographic (QCA) measurements were performed on the culprit lesion before intervention and after stenting (QAngioXA®; Medis medical imaging systems, Leiden, The Netherlands). Reference vessel diameter, minimum lumen diameter, lesion length, and diameter stenosis were measured.
OCT ANALYSIS
An independent OCT core laboratory (Heart Hospital, Tampere University Hospital, Tampere, Finland) performed the OCT image analysis. Quantitative analyses were performed using customised software (Heart Hospital, Tampere University Hospital, Finland) in combination with a proprietary off-line review workstation (St. Jude Medical) at 0.4 mm intervals as previously described10. Pre-stent thrombus volume (in mm3) and post-stent atherothrombotic volume (in mm3) were analysed over the arterial segment defined by the final stent length. Proximal and distal reference vessel areas (mm2) and diameters (mm) were measured at the largest lumen site within 5 mm outside the lesion’s edges. The mean value of proximal and distal measurements was used. Minimal lumen area (MLA) and minimal flow area were determined. Minimal flow area was defined as MLA – (attached thrombus area+free thrombus area). Percentage maximum lumen area stenosis was calculated as
![]()
Percentage flow area stenosis was calculated analogously.
Culprit lesion morphology of all cases was analysed by two independent observers (O.A. Kajander and T. Sheth). PR was defined as fibrous cap discontinuity usually associated with cavity formation14,15. IFC included both definite and probable erosions, the former defined as thrombus overlying a visualised intact cap and the latter as thrombus or luminal irregularity without a visible rupture site14,15. Nodular protruding calcium or a heavily calcified plaque usually with associated thrombus was defined as a calcified nodule. In addition, type of thrombus and location of rupture were assessed. Two independent observers (T. Sheth and O.A. Kajander) reviewed separately each patient’s OCT images. At a later time point the results were reviewed. The interobserver variability was assessed based on the independent reads. A consensus reading was used to resolve disagreement in any of the analysed variables.
For plaque composition analyses, the culprit lesion was defined by the number of consecutive frames containing ≥2 diseased quadrants. Plaque composition was analysed at 1 mm intervals over the length of the lesion using standard definitions14. At each interval, the number of quadrants with different plaque types (lipid, fibrotic or calcified) was recorded. These counts were summed over the length of the lesion to determine the total number of quadrants for each plaque type per lesion. Minimal fibrous cap thickness (FCT) was recorded as a mean of three measurements at the lesion site where the thinnest cap was observed. Two alternative limit values of FCT, ≤65 µm and ≤85 µm, were used to define the lesion as a thin-cap fibroatheroma (TCFA).
Statistics
Baseline variables were summarised as mean±standard deviation for continuous variables and n (%) for categorical variables. Baseline variables were compared between groups with the Pearson chi-square test for categorical variables and Student’s t-test for continuous variables. Clinical outcomes were compared between groups with the Fisher’s exact test. All OCT pre-stent and post-stent values were not normally distributed and were transformed to improve normality before statistical analysis. The t-test was used for significance testing of the transformed data. The two-sided type I error level was set at 5%. Of these variables, medians and interquartile ranges (IQR) are presented. If the transformed variable was also not normally distributed, non-parametric testing using the Mann-Whitney test was performed. Interobserver reliability of OCT culprit lesion diagnosis was assessed using the kappa statistic (κ).
Results
OCT imaging was available for 93 patients in the thrombectomy arm of the TOTAL-OCT substudy, of whom 70 had analysable culprit lesion morphology resulting in feasibility of 75.3%. Reasons for non-assessability included seven patients with balloon predilatation, excessive residual blood (seven cases) and excessive thrombus (four cases) (Figure 1).
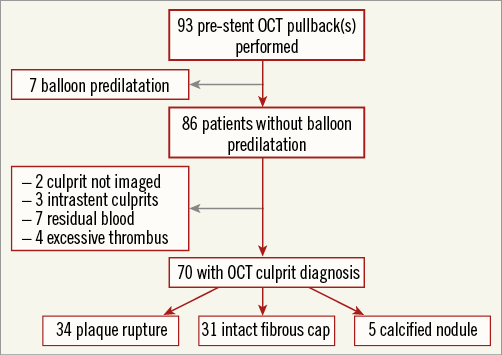
Figure 1. Patient flow chart of the study. OCT: optical coherence tomography
Culprit lesion morphology was PR in 34 (48.6%) patients, IFC in 31 (44.3%) patients and calcified nodule in 5 (7.1%) patients. Kappa values were 0.90 and 0.83 for PR and IFC, respectively. Due to the small numbers, patients with calcified nodules were not included in group-wise comparisons.
PR was located proximal to the MLA in 54.5% (n=18), at the MLA in 24.2% (n=8) and distal to the MLA in 21.2% (n=7) of patients. There were no clear differences in baseline patient characteristics between PR and IFC (Table 1). Greater than 70% resolution of ST elevation was not different (61.8 vs. 71.0%, PR vs. IFC, p=0.71) between groups. Two representative patient cases with PR and IFC are shown in Figure 2.
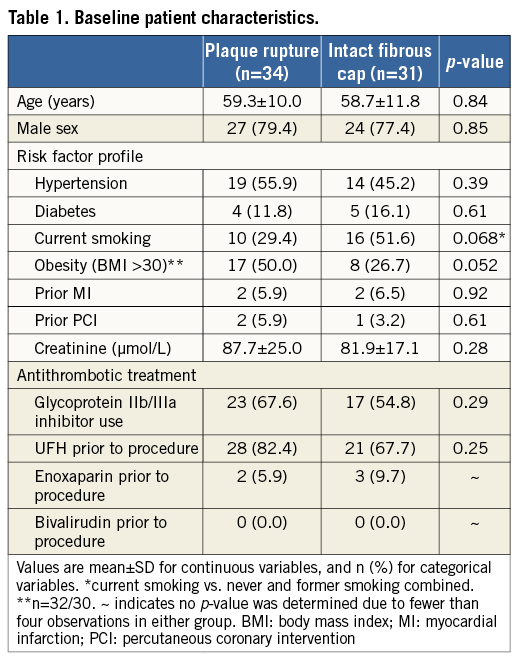
Figure 2. Representative post-thrombectomy angiographic and OCT images of two STEMI patients with plaque rupture and intact fibrous cap. A) Patient with angiographic lesion location in LAD (long red arrow) and plaque rupture at the proximal shoulder of a lipid-rich plaque in OCT. A cavity is displayed both in the cross-sectional (asterisk) and longitudinal (red arrow) views of the OCT image. B) Patient with culprit lesion in LAD (long white arrow) and an intact fibrous cap overlaid by luminal thrombus (arrowheads, white arrow) and no signs of plaque rupture in OCT. LAD: left anterior descending coronary artery; OCT: optical coherence tomography
ANGIOGRAPHIC FINDINGS
Pre-procedure angiographic results are shown in Table 2. Angiographic culprit lesion location was similar for PR and IFC. Pre-procedural TIMI 0 or 1 flow was present in 57.6% of patients with PR and 70.0% of patients with IFC (p=0.097). TIMI thrombus grade ≥3 was present equally (75.8 vs. 76.7%, p=0.93) in patients with PR and IFC. Reference vessel diameter was smaller (2.7±0.6 mm) in IFC than PR (3.0±0.8 mm, p=0.048). Other angiographic or PCI procedure details were not different between the groups.
OCT FINDINGS
Post-thrombectomy OCT measurements are presented in Table 3. For one patient in the PR group, the full volumetric pre-stent vessel and thrombus analyses were not completed due to low image quality. Reference vessel area (7.68 vs. 10.28 mm2, p<0.001) was lower in patients with IFC than PR. There was a trend for lower MLA (p=0.10) (Figure 3A) and minimal flow area (p=0.069) in patients with IFC than PR, but there were no differences in percentage lumen area stenosis or flow area stenosis. Lumen area stenosis <50% was observed in none of the patients with PR and in one patient with IFC (Figure 3B). Absolute thrombus volume was not statistically significantly different between the groups (3.11 mm3 [IQR: 1.31-7.87 mm3] vs. 2.39 mm3 [IQR: 0.85-5.26 mm3], p=0.17). Thrombus types did not differ between groups.
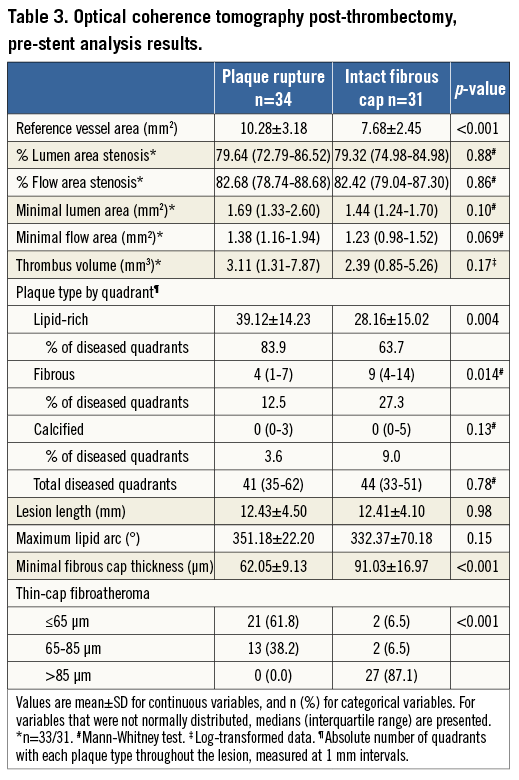
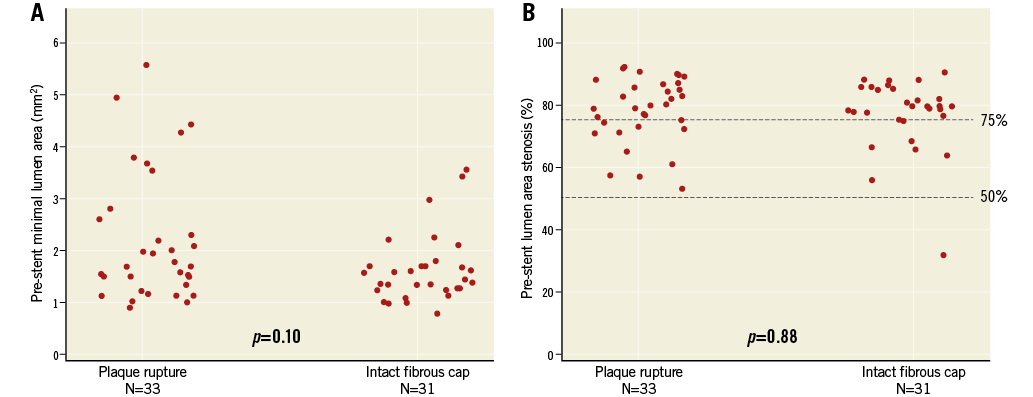
Figure 3. Scatter plots of OCT post-thrombectomy minimal lumen area (A) and percentage lumen area stenosis (B) in patients with plaque rupture and intact fibrous cap.
The number of lipid-rich quadrants in the culprit lesion was higher (39.12±14.23 vs. 28.16±15.02, p=0.004) (Figure 4A) and fibrous quadrants lower (4 [IQR: 1-7] vs. 9 [IQR: 4-14], p=0.014) in PR than in IFC (Table 3). However, lipid-rich quadrants were the predominant plaque type in both groups (83.9 vs. 63.7%, PR vs. IFC). In addition, the maximum lipid arc was comparable in the PR and IFC groups (351.18 vs. 332.37°, p=0.11). Culprit lesion minimal fibrous cap thickness (FCT) was 62.05±9.13 µm in patients with PR and 91.03±16.97 µm in patients with IFC (p<0.001) (Figure 4B). Minimal FCT ≤65 µm was observed in 21 (61.8%) patients with PR, compared with two (6.5%) with IFC, (p<0.001).
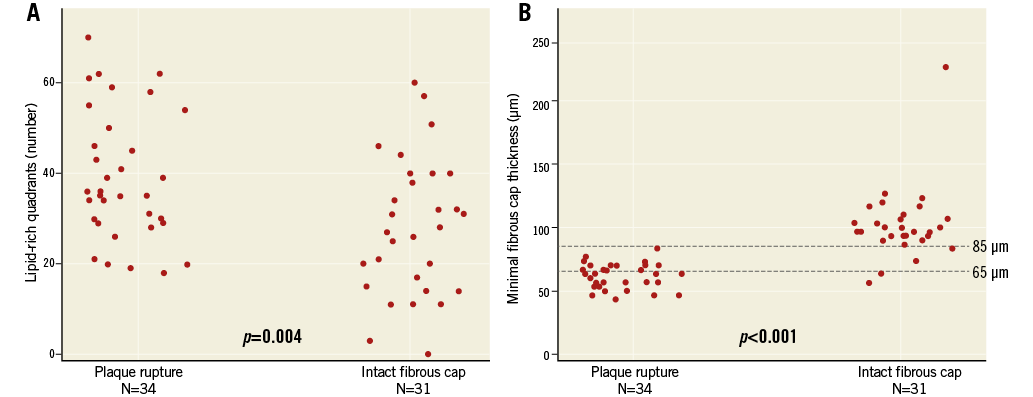
Figure 4. Scatter plots of number of quadrants with lipid (A) and minimal fibrous cap thickness (B).
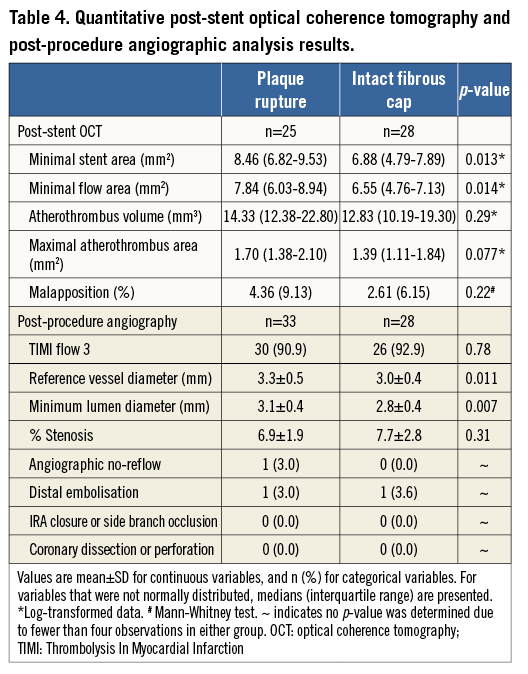
Following stent implantation, minimal stent area and post-stent minimal flow area were smaller in the IFC group, whereas in-stent atherothrombotic volume was not different between groups. The percentage of malapposed struts was comparable between groups (Table 4).
CLINICAL OUTCOMES
The rate of adverse clinical outcomes (a composite of cardiovascular death, recurrent myocardial infarction, cardiogenic shock and class IV heart failure) was low at six months and not different between PR and IFC (zero vs. two cases, p=0.22). There were two cases of recurrent myocardial infarction, which were in the IFC group.
Discussion
In a prospective study of STEMI patients treated with thrombectomy, mild residual stenoses were uncommon in IFC lesions. Although lipid content was lower than in PR lesions, lipid composed the majority of diseased segments in IFC. IFC lesions occurred in small vessels, but were otherwise indistinguishable by clinical or angiographic characteristics from PR lesions. The clinical event rate was low and there were no differences between IFC and PR patients.
STENOSIS SEVERITY
There has been increasing recognition that the culprit lesion for STEMI is usually severely stenotic. Angiographically severe stenoses have been shown in STEMI primary PCI patients, both after thrombectomy16 and after wiring of the culprit lesion17. However, the resolution of conventional angiography does not allow differentiating between thrombus and plaque at the MLA site18. A recent intravascular ultrasound (IVUS) study showed severe stenosis in STEMI patients and in the subgroup without plaque rupture, the only factor differentiating STEMI from other acute coronary syndromes being an MLA <2.3 mm219. In contrast to IVUS, OCT imaging allows differentiation of thrombus from plaque at the MLA site. Using this modality, we observed that stenoses in culprit lesions after thrombectomy are mainly composed of atherosclerotic plaque with a relatively minor contribution of thrombus, the quantity of which was not different between IFC and PR. The majority of IFC patients had post-thrombectomy OCT lumen area stenosis >75% at the culprit lesion and mild stenoses were rare. These findings are supported by previous OCT studies reporting similar degrees of stenosis in patients with IFC and PR6,7.
PLAQUE LIPID CONTENT
Data from previous studies have shown differences in culprit plaque lipid content between PR and IFC. In a previous OCT study, 100% of both IFC and PR were classified as lipid-rich7. Conversely, other OCT studies3,4 showed lipid plaque in 100% of PR, but only 43.3-43.6% of IFC. Lack of standardised methodology for lipid quantification by OCT may explain the controversial results. The criteria for lipid-rich plaque were ≥2 quadrants with lipid in any frame in one study7, a plaque with lipid arc >90° in the second study4 and not specifically described in the third3. Rather than analysing only the frame with the MLA or the maximum lipid arc, we evaluated plaque type through the length of the lesion. We showed a statistically significant excess number of lipid-rich quadrants in PR compared with IFC. However, lipid was the predominant plaque type in both lesion types and the maximum lipid arc was large in both groups. These findings probably account for the ability of NIRS to identify culprit lesions in STEMI despite not being able to identify plaque rupture at the lesion site9 and have implications for the prediction of future events based on OCT imaging. To the extent that IFC lesions lead to new events, strategies for prophylactic intervention that target only TCFAs are unlikely to eliminate all future STEMI events.
CLINICAL IMPLICATIONS
The mechanisms of coronary thrombosis in patients with IFC are not well known. In situ thrombus formation may be stimulated by systemic exposures such as smoking or local factors such as shear stress20. Our data on stenosis severity suggest that the presence of severe stenosis with small MLA may also be an important contributor. The smaller MLA could lead to occlusion with lower thrombus burden. In larger vessels, IFC lesions may be more likely to produce non-occlusive thrombi that do not present with ST-segment elevation, whereas PR lesions may be more thrombogenic and more likely to lead to vessel occlusion. Recently it has been suggested that, in ACS patients with IFC, there is only mild residual stenosis after successful treatment of thrombus8,21. Our prospective data, however, suggest that most IFC lesions are severely stenotic and that they have features of advanced atherosclerotic lesions with significant overlap observed in lipid content, lesion length and total number of diseased quadrants between IFC and PR.
Limitations
The present study has several limitations. The sample size of the study was relatively small. In addition, since performing OCT imaging requires clearing the vessel of blood, it was not possible to include patients in whom TIMI 2-3 flow could not be restored before stent implantation. Even after restoration of flow, insertion of the OCT catheter may have occluded the artery in some patients, leading to their exclusion from the study. For the same reason, thrombus volume measurement using OCT could only be performed after thrombectomy. Additionally, the use of a thrombectomy catheter prior to OCT imaging may have caused vessel trauma, which could have been interpreted erroneously as non-intactness of the fibrous cap. Persisting thrombus limits assessability of the culprit site, which in some studies has been overcome by performing a follow-up OCT after initial stabilisation of the patient. However, exclusion of patients was multifactorial and similar to other studies. Finally, the haemodynamic significance of post-thrombectomy stenoses was not studied and no follow-up OCT was performed.
Conclusions
In a prospective study of STEMI patients treated with thrombectomy, mild residual stenoses were uncommon in IFC lesions. Although lipid content was lower than in PR lesions, lipid composed the majority of diseased segments in IFC.
| Impact on daily practice In a prospective series, clinical and angiographic factors were not different between intact fibrous cap (IFC) and plaque rupture (PR), the two most common aetiologies of ST-elevation myocardial infarction (STEMI), as demonstrated by intravascular optical coherence tomography (OCT). Most STEMI patients have significant residual stenoses after thrombectomy, irrespective of the presence of PR or IFC at the culprit site. Both IFC and PR culprit lesions are advanced atherosclerotic lesions composed mainly of lipid-rich plaque, which may merit similar treatment strategies. |
Funding
The TOTAL-OCT substudy was supported by a grant from St. Jude Medical and a McMaster University Cardiology Division AFP Grant. The TOTAL trial was supported by grants from Medtronic and the Canadian Institute of Health Research, and CANNECTIN. O.A. Kajander was financially supported by the Competitive Research Funding of Tampere University Hospital, Finland. N. Pinilla-Echeverri has received a grant from the “Fundación Alfonso Martin Escudero”, Spain.
Conflict of interest statement
The authors have no conflicts of interest to declare.

