- virtual histology
- IVUS
- plaque rupture
Abstract
Aims: To characterise plaque phenotypes in the left main stem (LMS) and the proximal left anterior descending (LAD) coronary artery using virtual histology assisted intravascular ultrasound (VH-IVUS).
Methods and results: Patients with IVUS pullbacks including no less than the proximal 30 mm of the LAD and through the ostium of the left main were identified from a global IVUS registry. Plaque composition and phenotype frequency in the LMS and five consecutive non-overlapping 6 mm segments in the LAD were studied, resulting in six analysed segments per patient. There were 74 patients (72% male, mean age 65 years). The median LMS length was 5.4 mm (IQR 2.8–8.7 mm). The percent of fibrofatty plaque was greater in the LMS compared to the proximal LAD segments (27.9% [20.0-39.2] vs. 17.3% [12.2-23.1], p<0.001). Dense calcium and necrotic core content was less prevalent in the LMS compared to the LAD segments (2.5% [0.9-4.7] vs. 7.9% [4.1-12.3], p<0.001; and 8.0% [3.7-11.8] vs. 14% [9.2-17.9], p<0.001). The frequency of thin cap fibroatheroma (TCFA) was higher in the LAD compared with LMS (0% vs. 16.9% [4.9-34.5], p < 0.001,). Within the LAD, TCFA was most frequently observed in the second 6 mm segment, 12 mm from the ostium.
Conclusions: TCFA was present more frequently in the proximal LAD than LMS, supporting the notion that plaque rupture occurs in non-uniform locations throughout the coronary tree and preferentially spares the LMS.
Introduction
An acute coronary syndrome (ACS) usually occurs as a result of atherosclerotic plaque rupture of a coronary artery that previously was not severely narrowed1, creating a potent stimulus for platelet aggregation, thrombus formation and arterial occlusion2. Angiographic3 and intravascular ultrasound analyses (IVUS)4 have shown that the site of plaque rupture leading to an ACS tends to occur in predictable areas within the proximal third of the arteries and preferentially spares the left main stem (LMS) and more distal coronary segments.
Additional information from virtual histology –assisted IVUS (VH–IVUS) studies have extended these findings. Plaque composition appears to be non-uniformly distributed –principally high necrotic core content is localised to the proximal portion of the coronaries5. These findings can be further extended by performing plaque phenotyping. Therefore, the objective of this study was to describe the frequency of plaque phenotypes as defined by VH-IVUS in the LMS and proximal left anterior descending (LAD) in a prospective multinational VH-IVUS registry.
Methods
Patient selection
The VH-IVUS global registry enrolled patients >18 years old and without any contraindication to IVUS imaging who underwent diagnostic or interventional coronary procedures between 2004 and 2006 at 42 multinational centres. The ethics committee of each participating institution approved the protocol, and written informed consent was obtained from all patients. Dyslipidaemia was defined as total cholesterol level ≥200mg/dl, low-density lipoprotein cholesterol ≥100mg/dl, high-density lipoprotein cholesterol ≤50mg/dl, triglycerides ≥150mg/dl, or medication use. Hypertension was defined as systolic blood pressure ≥140mm Hg, diastolic blood pressure ≥ 90 mm Hg, or use of any antihypertensive drug. Diabetes mellitus was confirmed by diagnosis or use of anti-diabetic medications at study entry.
Patients underwent coronary angiography followed by IVUS at the discretion of the operator for one or more indications: 1)assessment of an indeterminate lesion, 2)investigation of a culprit lesion for optimal device sizing, 3)post-stent assessment, or 4)clarification of extent of disease.
In the present analysis, patients with analysable pre-intervention IVUS studies of the LMS (excluding the LMS bifurcation) and proximal 30 mm of the LAD, divided into equal 6 mm segments, were randomly selected (Figure 1). A length of 6 mm was chosen for the coronary segments located distal to the LMS carina based on the median length of LMS in the study cohort. Patients with incomplete pullback through the ostium of the LMS or <30mm of the proximal LAD were excluded.
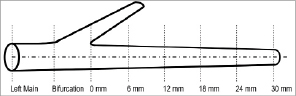
Figure 1. Intravascular ultrasound (IVUS) analysis. The proximal left anterior descending (LAD) coronary artery was divided into five 6 mm segments, the bifurcation was excluded and the left main stem (LMS) was analysed from the bifurcation to the ostium.
VH-IVUS image acquisition and analysis
Coronary vessels were imaged with 20MHz IVUS catheters (Eagle Eye® Gold; Volcano Corp., Rancho Cordova, CA, USA) during continuous motorised pullback at 0.5mm/s (R-100TM and TrakBack®II; Volcano Corp.) with qualitative IVUS analysis performed according to the American College of Cardiology (ACC) expert consensus on IVUS6. Plaque volume was derived using the Trapezoidal rule7,8 as follows: (external elastic membrane [EEM] volume-lumen volume). Plaque burden was defined as ([EEM area-lumen area] / EEM area) x 100. Geometric and compositional data for each frame was calculated using VH-IVUS software (pcVH V2.2; Volcano Corp.), a validated method9,10 that utilises the mathematical technique of autoregressive modelling to classify IVUS-radiofrequency data into one of four colour-coded plaque components: 1)fibrous (green), 2)fibrofatty (light-green), 3)dense calcium (white), and 4)necrotic core (red)11-13. VH-IVUS borders were drawn by technicians at the Cleveland Clinic Department of Biomedical Engineering (Cleveland, OH, USA) or the Cardiovascular Research Foundation (New York, NY, USA) and verified at Cardialysis (Rotterdam, The Netherlands).
VH–IVUS phenotype plaque classification
VH-IVUS frames were assigned a plaque type by an automated pixel detection algorithm based on a modified histopathological classification scheme14 and expert consensus guidelines15 for analysing and interpreting VH-IVUS images (Figure 2). Interobserver variability in lesion type classification is removed by the algorithm. A confluent area of composition was defined as a circular area of the same tissue type for a minimum diameter of approximately 12pixels or 0.08mm2 on a 400×400 VH-IVUS image. Non-atherosclerotic intimal deposits of plaque <600µm on any VH-IVUS frame were classified as intimal medial thickening. Plaque >600µm thick was assigned to one of four atherosclerotic phenotypes as follows: (1)pathological intimal thickening, predominantly fibrous tissue with or without >15% fibrofatty tissue and without either confluent necrotic core or confluent dense calcium; (2)fibrocalcific plaque confluent dense calcium without confluent necrotic core; (3) fibroatheroma, confluent necrotic core either not at the lumen or not exceeding 14 pixels along the circumference of the lumen on 3 consecutive frames; (4) thin cap fibroatheroma (TCFA), plaque burden (PB) >50% and confluent necrotic core extending >14pixels along the circumference of the lumen on three consecutive frames.
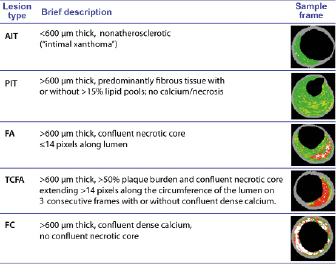
Figure 2. Intravascular ultrasound virtual histology (IVUS–VH) classification system for plaque phenotypes. AIT: adaptative intimal thickening; PIT: pathological intimal thickening; FC: fibrocalcific; FA: fibroatheroma; TCFA: thin-cap fibroatheroma
Statistical analysis
The statistical analysis was performed using the SAS 9.2 software package (SAS Institute, Cary, NC, USA). Continuous and categorical variables are presented as mean ± standard deviation (SD) and median and interquartile range (IQR) as appropriate. Grayscale IVUS, plaque composition and lesion phenotype data were compared between the left main, LAD and different LAD segments using the Kruskal-Wallis test (continuous data) and the Chi-square test (categorical data). A p value <0.05 was considered significant.
Results
There were 990 patients with complete analysable IVUS images. Among 100 patients who underwent an IVUS evaluation including the proximal LAD and were selected at random for this study, 26 were excluded from the analysis due to short (<30mm) IVUS pullback in the LAD or lack of complete pullback through the ostium of the left main. Thus, 74 patients (72% male; mean age 65 years) constituted the final population. Baseline characteristics are displayed in Table 1. The median LMS length was 5.4mm (IQR 2.8-8.7mm).
Geometric IVUS measures
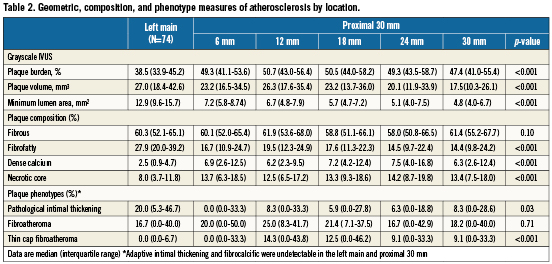
Compared with the LMS, the proximal LAD had greater PB over the mean length as well as in each 6mm segment. The plaque volume in the LMS was greater than each LAD segment or the mean plaque volume for the entire LAD pullback length. In contrast, the minimal luminal area (MLA) in the LMS was greater than the median MLA over the proximal 30 mm of the LAD and each individual LAD segment (Table 2).
VH-IVUS plaque composition
Fibrous tissue was the predominant plaque component of the LMS and LAD with similar percentages in both locations. Fibrofatty plaque composition was larger in the LMS compared to the LAD segments. The percentages of dense calcium and necrotic core were much lower in the LMS compared to the LAD. The necrotic core content peaked at 14.2% in the fourth 6mm LAD segment (Table2).
VH-IVUS plaque phenotype classification
Adaptive intimal thickening and the more advanced fibrocalcific plaque type were absent in the LMS or proximal 30 mm of the LAD. The distribution of fibroatheroma plaques was similar in the LMS and LAD. Pathological intimal thickening was more common in the LMS than the proximal LAD. TCFA was undetectable in the LMS and first 6mm LAD segment, its frequency peaked at 14.3% in the second 6mm and tapered off in the remaining 18 mm LAD segment (Figure 3).
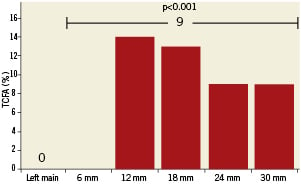
Figure 3. Thin cap fibroatheroma (TCFA) frequency distribution. The frequency of TCFA is presented in the left main stem as well as the proximal 30 mm, divided by 6 mm segments. TCFA was present in 9% of the entire proximal 30 mm.
Discussion
The main findings of the present study are: 1)The LMS has significantly lower PB than the proximal LAD; 2)The volume of necrotic core occurs at much lower frequencies in the LMS compared to the LAD; and 3)TCFA was absent in the LMS and the frequency increased in the proximal LAD and peaked in the second and third 6 mm LAD segments, respectively.
Earlier angiographic studies of the location of plaque rupture causing STEMI suggest that the sites of rupture are clustered in coronary segments within the first 40mm of the LAD in 90% of the cases3. These findings are consistent with IVUS analyses that showed that LAD plaque ruptures are located 10-40 mm from the LAD ostium in 83% of cases4. In pathology studies, plaque rupture distribution appears to be directly related to the distribution of coronary atherosclerosis16, plaque composition, and TCFA17, with a greater burden of atherosclerosis and increased frequency of necrotic core and TCFA clustered in more proximal coronary segments but preferentially sparing the LMS.
Our results are consistent with previous analyses where the mean PB of the LMS was approximately 34% but had minimal necrotic core5. We extend these findings by identifying that TCFA was absent in the LMS which may explain the lower incidence of LM STEMI in clinical practice or represent a selection bias as complete thrombotic occlusion of the LMS as a result of plaque rupture may be more often fatal, which could explain the high frequency of LMS TCFA in sudden cardiac death patients in autopsy studies18. On the other hand, ruptured LMS plaques presenting to the cardiac catheterisation laboratory have a sub-occlusive thrombi that rarely compromises the lumen of the LMS19. Therefore, the presence of TCFA in patients with angiographically mild coronary lesions may serve as a predictor of long term adverse cardiovascular events as recently suggested by the PROSPECT trial20.
The main limitations of this retrospective analysis are its small sample size, lack of post procedural clinical follow-up, which hampers our ability to link VH-IVUS findings with clinical outcomes data; and serial VH-IVUS measurements. However, recent analyses demonstrated a positive correlation between the risk of clinical events derived from three risk-score algorithms for primary prevention and the extent of plaque progression in the LMS measured by serial IVUS, which translates into stenosis progression with increasing clinical risk21. It is also important to acknowledge that we excluded the site of the LMS bifurcation segment in this analysis. IVUS analyses have demonstrated that a high percentage of patients with an angiographically normal LMS bifurcation have atherosclerosis at the bifurcation site22. The distribution of atherosclerosis is diffuse rather than focal and both sides of the flow divider (carina) are usually spared23. Also, percent necrotic core was greatest at the bifurcation compared with the proximal segment of the left main, suggesting a non-uniform distribution at the bifurcation24.
Conclusions
TCFA was present more frequently in the proximal LAD than LMS, supporting the notion that plaque rupture occurs in non-uniform locations throughout the coronary tree and preferentially spares the LMS.
Acknowledgements
We thank Joseph Murphy and Jose Aceituno for publication assistance.
Conflict of interest statement
Dr. Marso reports no personal conflict of interest within the past 12 months. His institution has received consulting fees from The Medicines Company, Novo Nordisk, and Abbott Vascular; research support from The Medicines Company, Amylin Pharmaceuticals, Boston Scientific, Volcano Corporation, and Terumo Medical. Dr. Pieper is a consultant for Volcano Corporation. Dr. Lindsey is a consultant for Novo Nordisk. Dr Stolker is on the speaker’s bureau for AstraZeneca. The other authors have no conflicts of interest to declare.

