Abstract
Aims: We report the intravascular ultrasound (IVUS) analysis of plaque distribution in left anterior descending (LAD) artery/first diagonal (D1) and distal left main coronary artery (LMCA) bifurcation lesion location.
Methods and results: We reviewed 58 angiograms of LAD/D1 bifurcation lesions with pre-intervention IVUS of both the LAD and D1 and compared these data to a corresponding cohort (n=81) of LMCA bifurcations, dividing each bifurcation into three segments: MV (main vessel), MB (main branch distal to the carina), and SB (side branch). In the LAD/D1 cohort, D1 (SB) had less calcium and a smaller plaque burden compared to the other two segments. Continuous plaque from the LAD proximal to the carina (MV) into the LAD distal to the carina (MB) was seen in 90%, from the MV into the SB in 72%, and from the MV into both the MB and SB in 62%. In the LMCA cohort, ostial left circumflex (LCX) (SB) had less calcium and a smaller plaque burden compared to the distal LMCA (MV) and ostial LAD (MB). Continuous plaque from MV to MB was seen in 96%, from MV to the SB in 78%, and from MV to both branches in 74%.
Conclusions: The IVUS analysis of the LAD/D1 and LMCA bifurcations revealed that most lesions had diffuse plaques extending from the MV into the MB with the SB having the least amount of calcium and the smallest plaque burden, regardless of location.
Introduction
Percutaneous coronary intervention of a bifurcation lesion continues to be a challenge. Restenosis rates after the implantation of bare metal stents are unacceptably high regardless of the technique1,2. Since the advent of drug-eluting stents, restenosis rates have decreased both in retrospective analyses and in dedicated prospective studies3-5; however, they remain higher in bifurcation lesions than in non-bifurcations6,7. Furthermore, in recent bifurcation studies cumulative major adverse cardiac event rates are higher in left main coronary artery (LMCA) bifurcation lesions (20% to 30%) compared to non-LMCA bifurcation lesions (10% to 20%). In particular, restenosis at the left circumflex (LCX) ostium after a two-stent technique is higher than at a non-LMCA side branch (SB) ostium8-12. We hypothesised that there would be differences in atherosclerosis morphology and distribution between LMCA and non-LMCA bifurcation lesion locations that might help to explain these findings. Therefore, we reviewed and compared pre-intervention intravascular ultrasound images obtained from both the main vessel and side branch pre-intervention at two locations: the distal LMCA and the left anterior descending artery/first diagonal branch (LAD/D1) bifurcations.
Methods
We derived the patients in this study from two sources. From the database of the Showa University Northern Yokohama Hospital (Yokohama, Japan), we identified 72 patients with angiographically significant lesions of the LAD/D1 bifurcation (angiographic diameter stenosis ≥50% in one or more vessels <5 mm from the bifurcation site) who underwent pre-interventional intravascular ultrasound assessment of both the LAD and the D1 arteries and who then underwent stent implantation between July 2004 and April 2010. Patients with in-stent restenosis, acute myocardial infarction, and poor image quality were excluded (n=14). The main vessel (MV) was the LAD proximal to the carina, the main branch (MB) was the LAD distal to the carina, and the SB was D1.
The comparison group included 81 patients with a significant distal LMCA lesion (angiographic diameter stenosis ≥50% in one or more vessels <5 mm from the bifurcation site) who underwent stent implantation after intravascular ultrasound imaging of both the LAD and LCX. These represent a subgroup of a previously reported cohort of 140 patients that also included 59 patients without significant distal LMCA bifurcation disease and who were excluded from the current analysis13. In this LMCA cohort the MV was the LMCA, the MB was the LAD, and the SB was the LCX.
Patient demographics were confirmed by hospital chart review at the time of the procedure. Coronary risk factors included diabetes mellitus (diet-controlled, oral agent, or insulin-treated), hypertension (medication-treated only), and hypercholesterolaemia (medication-treated or >240 mg/dL).
All angiograms were assessed before the introduction of the coronary guidewire by experienced observers without knowledge of clinical or intravascular ultrasound data. All segments within 10 mm from the LAD/D1 and LMCA bifurcation were analysed with a computer-assisted, automated edge-detection algorithm (CMS; Medis, Leiden, The Netherlands). The diameters of the proximal angiographically normal segments (for the MV) and distal angiographically normal segments (for the MB and SB) were used as the reference. Minimum lumen diameter at end diastole was measured in multiple projections, and results from the worst view were recorded. Qualitative analysis of the LAD/D1 and LMCA bifurcation was assessed as proposed by Medina et al 14 where “1” indicated the presence of stenosis and “0” indicated the absence of stenosis in three segments separated by commas: the MV, the MB, and the SB in this order. Bifurcation angles were defined as angles between the MB and SB.
All studies were performed before intervention and after 200 µg of intracoronary nitroglycerine; images were acquired using a commercially available intravascular ultrasound system (40 MHz mechanical transducer; Boston Scientific Corp, Natick, MA, USA). IVUS evaluation was performed on all three segments of each bifurcation: the MV, MB, and SB. The probe was advanced into the distal MB and SB, and an imaging run was performed back to the proximal MV at an automatic pullback speed of 0.5 or 1.0 mm/sec. Intravascular ultrasound images were recorded onto digital media for offline analysis.
Qualitative and quantitative analyses were performed by independent observers according to the criteria of the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement, and Reporting of Intravascular Ultrasound Studies15. Because the MV was studied twice (i.e., during pullbacks from the MB and SB), the best images were selected for analysis after evaluation of both pullbacks. An intravascular ultrasound lesion was defined as a plaque burden ≥40%. The carina cross-section was the frame immediately distal to the take-off of the SB <5 mm from the bifurcation point in which both ostia of the MB and SB could be visualised as a figure-of-eight shape. The distal MV was the frame immediately proximal to the carina in which the vessel had a circular or oval shape. Involvement of the carina was carefully evaluated.
All studies were reviewed frame by frame during the cardiac cycle; and, with the use of planimetry software (echoPlaque™; INDEC Systems, Inc., Mountain View, CA, USA), quantitative IVUS analysis was performed every 1mm in the three selected segments including the external elastic membrane cross-sectional area (CSA), lumen CSA, plaque and media (external elastic membrane minus lumen) CSA and thickness, and plaque burden (plaque and media divided by external elastic membrane). The minimum lumen area site –the slice with the smallest lumen and the largest plaque and media area– was selected for the stenosis measurement. Lengths in millimetres were calculated from the pullback speed of the transducer. Calcium was brighter than the adventitia with acoustic shadowing of underlying tissue; the maximum arc (in degrees) and length (in millimetres) for the entire lesion were measured. A calcium length index was then calculated as total calcium length divided by analysed length. Reference segments were defined as the most normal-looking (largest lumen with smallest plaque burden) cross-sections proximal and/or distal to the lesion, as appropriate16,17. A remodelling index was calculated as lesion divided by the distal reference external elastic membrane for the MB and SB and lesion divided by the proximal reference external elastic membrane CSA for the MV. Negative remodelling was defined as a remodelling index <1.0. Circumferential plaque with carina involvement was defined as “concentric” plaque distribution. Volumetric data including external elastic membrane, lumen, and plaque and media volume were calculated from Simpson’s rule and normalised for analysis length.
With regard to geographical distribution of plaque in LAD/D1 bifurcation lesions, we analysed plaque location according to the classification that we adopted in the previous study of distal LMCA bifurcations13. In keeping with the Medina scoring system, the MV arteries were classified as 0/0 (no disease), 1/0 (disease opposite the flow divider on the side of the MV), 0/1 (disease opposite the flow divider on the side of the SB), and 1/1 (disease opposite the flow divider on both the MB and SB sides). The MB and SB arteries were classified as diseased or disease-free. In addition, we classified lesions into (1) diffuse disease that was continuous and concentric from the MV to the MB or SB or (2) focal disease that was not continuous and concentric.
Statistical analysis was performed with StatView® 5.0 (SAS Institute Inc., Cary, NC, USA). Continuous data were reported as mean±1SD if normally distributed. The three segments were compared with analysis of variance (ANOVA). Paired t-tests were used to compare continuous variables within the same segment of the bifurcation. Unpaired t-tests were used to compare different arterial segments. Categorical variables (presented as frequencies) were compared by χ2 statistics or Fisher’s exact probability test. A p value of <0.05 was considered statistically significant.
Results
Clinical characteristics are shown in Table 1. There were no significant differences when comparing patients with distal LAD/D1 bifurcation lesions versus patients with LMCA bifurcation lesions.
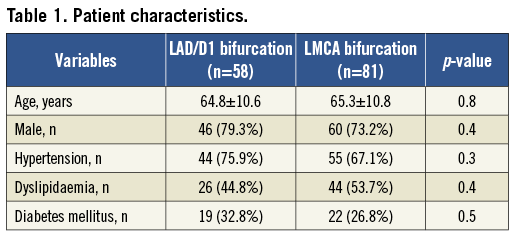
Medina classifications are shown in Table 2; there was a similar distribution between the two groups (p=1.0). True bifurcation lesions (Medina 1,1,1; 1,0,1; or 0,1,1) accounted for 52% of the LAD/D1 group and 47% of the LMCA group. On the one hand, we observed Medina “1”, but an intravascular ultrasound plaque burden of <70% in 5% of LAD/D1 lesions versus 26% of LMCA lesions in the MV (p<0.01); however, this was seen in 33% of MB in the LAD/D1 versus 40% of the MB in LMCA (p=0.68) and in 48% of SB in LAD/D1 versus 52% of SB in LMCA (p=0.81). On the other hand, a Medina “0” and PB of ≥70% was seen in 9% of LAD/D1 (versus 6% of LMCA) in the MV, 9% of LAD/D1 (versus 4% of LMCA) in the MB, and 2% of LAD/D1 (versus 2%) of the SB.
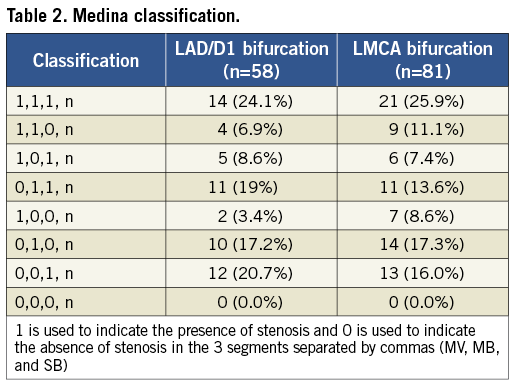
Table 3 demonstrates qualitative comparative analysis (QCA) in the two groups. The average angle between the MB and SB was significantly larger in the LMCA group than in the LAD/D1 group (97.7±19.1° vs. 51.5±19.5°; p<0.001). The MV had the largest average reference vessel diameter, and SB had the smallest average reference diameter in both groups. However, in the LMCA group the reference vessel diameter and the minimal lumen diameter were not statistically different between the MB and the SB.
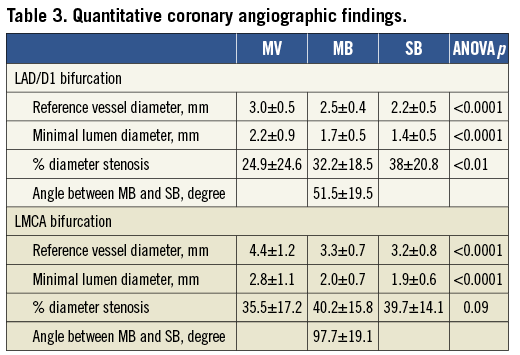
Quantitative volumetric intravascular ultrasound analyses in the LAD/D1 and LMCA groups are shown in Table 4. In the LAD/D1 group distances from the carina to the minimal lumen sites were similar among the three segments. However, in the LMCA group, the minimum lumen site was located closer to the carina in the MV segment compared to the MB and SB. The SB in both groups had a smaller calcium arc and calcium length index compared to the MV and MB. The SB had the smallest plaque burden (p<0.0001) with the greatest degree of negative remodelling (p<0.01) among the three segments in the LAD/D1 group. In the LMCA group the SB had the smallest plaque burden (p<0.0001); however, remodelling indexes were similar among the three segments (p=0.25).
Figure 1 shows the distribution of plaque burden in bifurcation lesions millimetre by millimetre in both groups. Plaque burden was greatest at the carina and decreased with the distance from the carina in all three segments in both the LAD/D1 and LMCA groups.
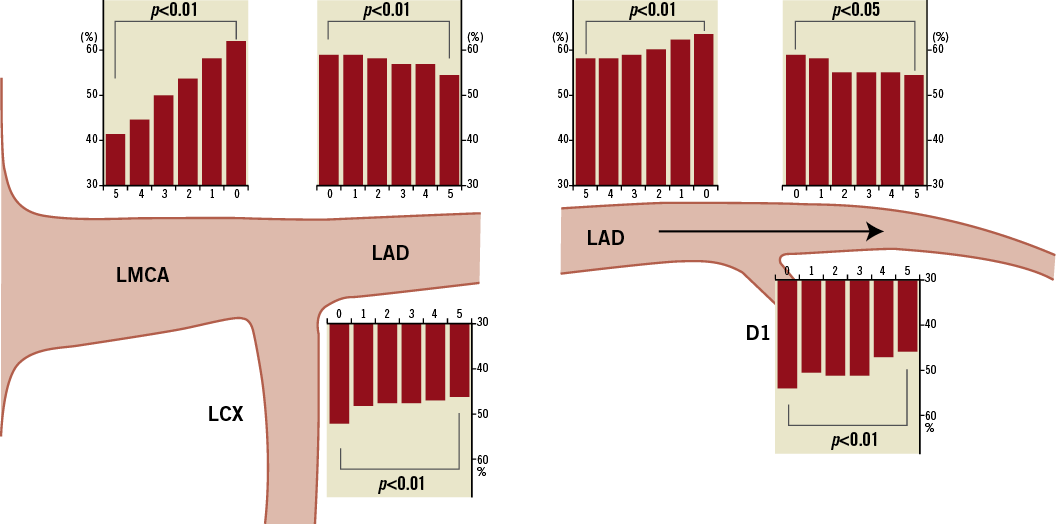
Figure 1. Plaque burden (%) every 1 mm at the distal LMCA bifurcation lesion location (left panels) and the LAD/D1 bifurcation lesion location (right panels). The distance proximal or distal to the carina (in mm) is indicated on each “x-axis.”
Figure 2 shows the comparison of the maximum calcium arc and calcium length index and volumetric plaque burden among the three segments. Values related to calcium were significantly less in the SB compared to the MV and MB in both the LAD/D1 and LMCA groups. However, at the LMCA, but not the LAD/D1 bifurcation location, calcium length index and plaque burden were significantly less in the MV than in the MB. Comparison between the LAD/D1 and LMCA groups revealed that MV plaque burden was less at the LMCA location (i.e., in the LMCA proximal to the carina) than at the LAD/D1 location (i.e., in the LAD proximal to the carina).
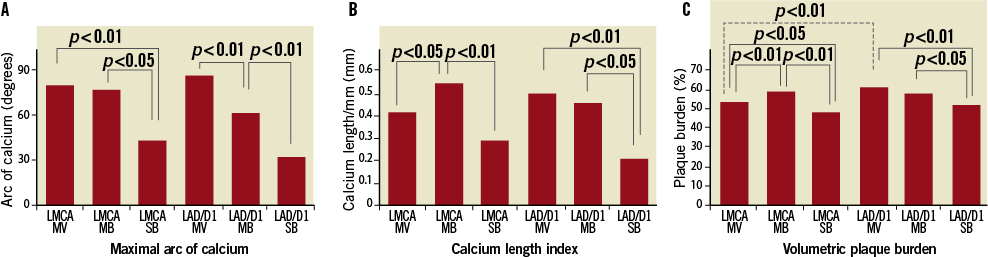
Figure 2. Maximal arc of calcium (A), calcium length index (B), and volumetric plaque burden (C) showing that the side branch had less calcium and plaque burden in both groups.
Our proposed intravascular ultrasound classification13 of plaque distribution for bifurcation lesions is shown in Figure 3. As many as 90% of all LAD/D1 bifurcations were classified as 1/1,1,1; 1/0,1,1; or 1/0,1,0. In other words plaque was continuous from the MV (proximal LAD) to the MB (LAD distal to the carina) in 90% of LAD/D1 bifurcation lesions. Plaque also extended from the MV into the SB (D1) in 72%. Plaque extended from the MV into both the MB and SB in 62%. Isolated plaques (with plaque thickness of <0.5 mm at the adjacent carina) were uncommon and were localised in the MV in two lesions, the MB in three lesions, and the SB in seven lesions. Plaque was distributed predominantly opposite the flow divider (n=48); however, the flow divider site was diseased in four cases.
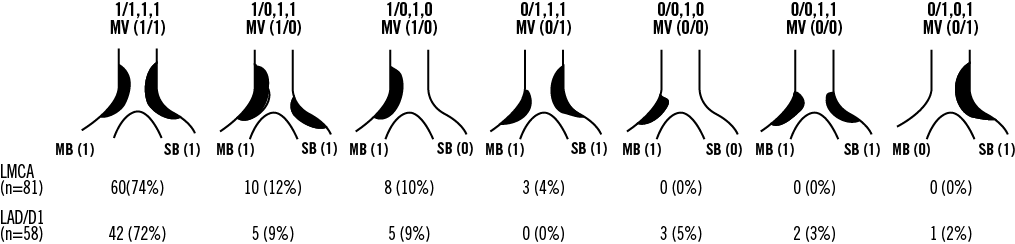
Figure 3. Spatial distribution of the plaque in LMCA and LAD/D1 bifurcation lesion locations indicating continuous plaque from the MV to MB in >90% of lesions in both groups.
The LMCA cohort was similar to the LAD/D1 cohort (Figure 3) with plaques only located opposite the flow divider. Plaque was continuous from the MV (LMCA) to the MB (LAD) in 96% of LMCA bifurcation lesions, from the MV into the SB (LCX) in 78%, from the MV into both the MB and SB in 74%.
Among lesions with both MV and MB involvement, the frequency of concentric plaque distribution –plaque that involves the entire circumference of both the MB and SB sparing only the carina or flow divider– was similar between LMCA and LAD/D1 groups (51% vs. 52%; p=0.88).
Discussion
We used pre-intervention intravascular ultrasound of the LAD/D1 and LMCA bifurcation, including pullback from both the MB and the SB into the MV, in all patients, to compare plaque distribution at two different bifurcation lesion locations. In summary, both locations had similar quantitative and qualitative intravascular ultrasound analyses regarding plaque distribution in contrast to our hypothesis that there would be differences between these two bifurcation locations.
Plaque burden but not lumen CSA has a positive correlation with calcium deposition18,19. In the current study lesions with more plaque tended to have more calcification. As a result there was more calcification in the proximal part of the LAD than in the other segments, while in the LMCA group the MV had less calcification in association with less plaque burden. This is consistent with a previous report by Schmermund et al using electron beam computed tomography20.
The geographical patterns of plaque distribution in the LAD/D1 and LMCA bifurcation lesions were similar in the current study. In addition, most plaques extended from the MV into the MB, and solitary plaques were consistently rare. Atherosclerosis has a predilection for the outer walls of bifurcations, and involvement of the flow divider is minimal or absent21-23. In the current study no patient in the distal LMCA bifurcation group had plaque involving the carina or flow divider consistent with previous pathologic and clinical studies13,24. Conversely in the LAD/D1 cohort in the current study, there were four lesions with plaque located on the carina side. Three of the four LAD/D1 lesions had a septal branch close to the D1. Septal perforators in all three cases were within 1.5 mm of the LAD/D1 bifurcation and had a calibre of ≥1.5 mm that might explain these exceptions. This implies that the haemodynamics, shear stress, and other local factors are similar between the two bifurcation lesion locations.
Bifurcations lesions are caused by haemodynamic turbulence and low shear stress25-28. Low shear stress may lead to reduced bioavailability of nitric oxide and endothelial dysfunction28,29. In addition, low shear stress may promote low-density lipoprotein cholesterol uptake30. In recent studies three-dimensional computational models have been employed to visualise coronary artery shear stress27,31,32. Our data correspond well with these models that show low shear stress areas opposite the flow divider (carina) regardless of bifurcation lesion location. While we expected regional differences between the LMCA and LAD/D1 bifurcations, in this IVUS study we observed only similar findings in the two groups.
Pre-interventional coronary morphology is a significant factor in prognosis after percutaneous coronary intervention (PCI), affecting both early and late clinical outcomes33,34. The angiogram may be misleading, especially in bifurcation lesions13. An angiographic stenosis does not always mean a large plaque burden, especially when the lesion is located at the SB origin. In our study of LAD/D1 (LMCA) bifurcation lesions, a Medina “1” in the setting of a plaque burden <70% was more common than a Medina “0” in the setting of a plaque burden >70%. Thus, false positives are more common than false negatives. The current intravascular ultrasound data show that >90% of distal LMCA and LAD/D1 bifurcation have significant plaques extending from the MV to the MB regardless of angiographic appearance, which may help us in the planning of bifurcation lesion treatment. Moreover, our data support the current trend to treat bifurcation lesions by extending the MV stent into the MB35.
The LAD/D1 data was from a single-centre retrospective study and patient selection was based on the availability of pre-intervention intravascular ultrasound data from both the LAD and the D1 arteries. The LMCA bifurcation cohort was part of a multicentre retrospective analysis. The usage of intravascular ultrasound, and the decision to image both vessels, were at the discretion of the operator. Thus, there was inherent heterogeneity and potential selection bias between two groups including race, vessel size, and bifurcation angles.
Conclusions
At the LAD/D1 bifurcation lesion location the SB had less calcium, a smaller plaque burden, and more negative remodelling compared to the MB. This finding was similar to the LMCA bifurcation lesion location. In addition, IVUS analysis of plaque distribution showed continuous plaque from the MV into the MB in >90% of LAD/D1 lesions, also similar to the distal LMCA bifurcation location.
Conflict of interest statement
A. Maehara and G.S. Mintz receive grants/research support from Boston Scientific Corporation and Volcano Corporation. G.S. Mintz is also a consultant for both Boston Scientific Corporation and Volcano Corporation. C. Oviedo is an employee of Volcano Corporation. M.B. Leon is a member of the advisory boards for Boston Scientific Corporation. G.W. Stone is a consultant for Volcano Corporation. J.W. Moses is a consultant for Boston Scientific Corporation. M. Ochiai is a member of the speakers’ bureau of Boston Scientific Corporation. All other authors have no conflicts of interest to declare.

