Abstract
The Bifurcation Academic Research Consortium (Bif-ARC) project originated from the need to overcome the paucity of standardization and comparability between studies involving bifurcation coronary lesions. This document is the result of a collaborative effort between academic research organizations and the most renowned interventional cardiology societies focused on bifurcation lesions in Europe, the United States, and Asia. This consensus provides standardized definitions for bifurcation lesions; the criteria to judge the side branch relevance; the procedural, mechanistic, and clinical endpoints for every type of bifurcation study; and the follow-up methods. Considering the complexity of bifurcation lesions and their evaluation, detailed instructions and technical aspects for site and core laboratory analysis of bifurcation lesions are also reported. The recommendations included within this consensus will facilitate pooled analyses and the effective comparison of data in the future, improving the clinical relevance of trials in bifurcation lesions, and the quality of care in this subset of patients.
Introduction
Historically, a coronary bifurcation lesion has been described as “a coronary artery narrowing occurring adjacent to, and/or involving the origin of a significant side branch (SB).” This description facilitates discussion of when a SB is significant, and thereby when sufficient flow in both vessels should be preserved and secured during treatment.1
Historical and on-going studies on the management of bifurcation lesions have primarily focused upon provisional vs upfront 2-stent techniques, and how to optimally perform these to preserve flow in both branches and minimize long-term adverse events (Supplemental Table 1). However, emerging developments in techniques, and devices such as drug-coated balloons (DCB), scoring balloons, dedicated bifurcation devices, and lesion-modification approaches using intravascular lithotripsy, and rotational and directional atherectomy, all now merit assessment in the treatment of bifurcation lesions (Supplemental Table 2). Nevertheless, their management (from patient selection to technical or procedural aspects and the definition of relevant clinical outcomes) requires additional consideration and standardization to enable accurate reproducibility in future dedicated studies, which thus far has not been achieved (Supplemental Table 3). In this regard, we need to clearly define the relevance of a SB, the acute technical/procedural success, and long-term clinical outcomes. These standardized definitions should incorporate, among other details, the angiographic classification, as well as the added value of intravascular imaging, the added value of new noninvasive (image-based) vs invasive methods of functional lesion evaluation, the different types of treatment (medical vs surgical or percutaneous revascularization), and the post-revascularization antiplatelet regimen (type, intensity, duration).
As part of the Academic Research Consortium program, our goal is to create consistent, practical, and reproducible terminology for the methodological approach and endpoints of clinical trials involving coronary bifurcation lesions, thereby improving the clarity of study design and reporting, facilitating pooled analyses and the effective comparison of data in the future. The overall objective is to improve the clinical relevance of trials in bifurcation lesions, hence improving the quality of care in this subset of patients (Figure 1).
Figure 2 depicts the general requirements to be met before undertaking any coronary bifurcation study.
Specifically, this position paper aims to define:
1) A classification for coronary bifurcation lesions from the perspective of symptoms, anatomy, function, and prognosis;
2) The specific technical details relevant to treatment of bifurcation lesions that must be captured;
3) Procedural, mechanistic (anatomical and functional), clinical, and cost-effectiveness endpoints;
4) Patient-, device-, vessel-, and bifurcation-oriented endpoints;
5) Patient-, site-, and central adjudication–reported endpoints;
6) Analytical plan related to intention-to-treat, per- protocol, and as-treated analyses (with the option of sham treatment);
7) Statistical handling of the different methods for analyzing composite endpoints (eg, competing risk, win ratio, negative binomial, Andersen-Gill, Wei-Lin-Weissfel); and
8) Optimal duration of follow-up, considering the study type and objectives.
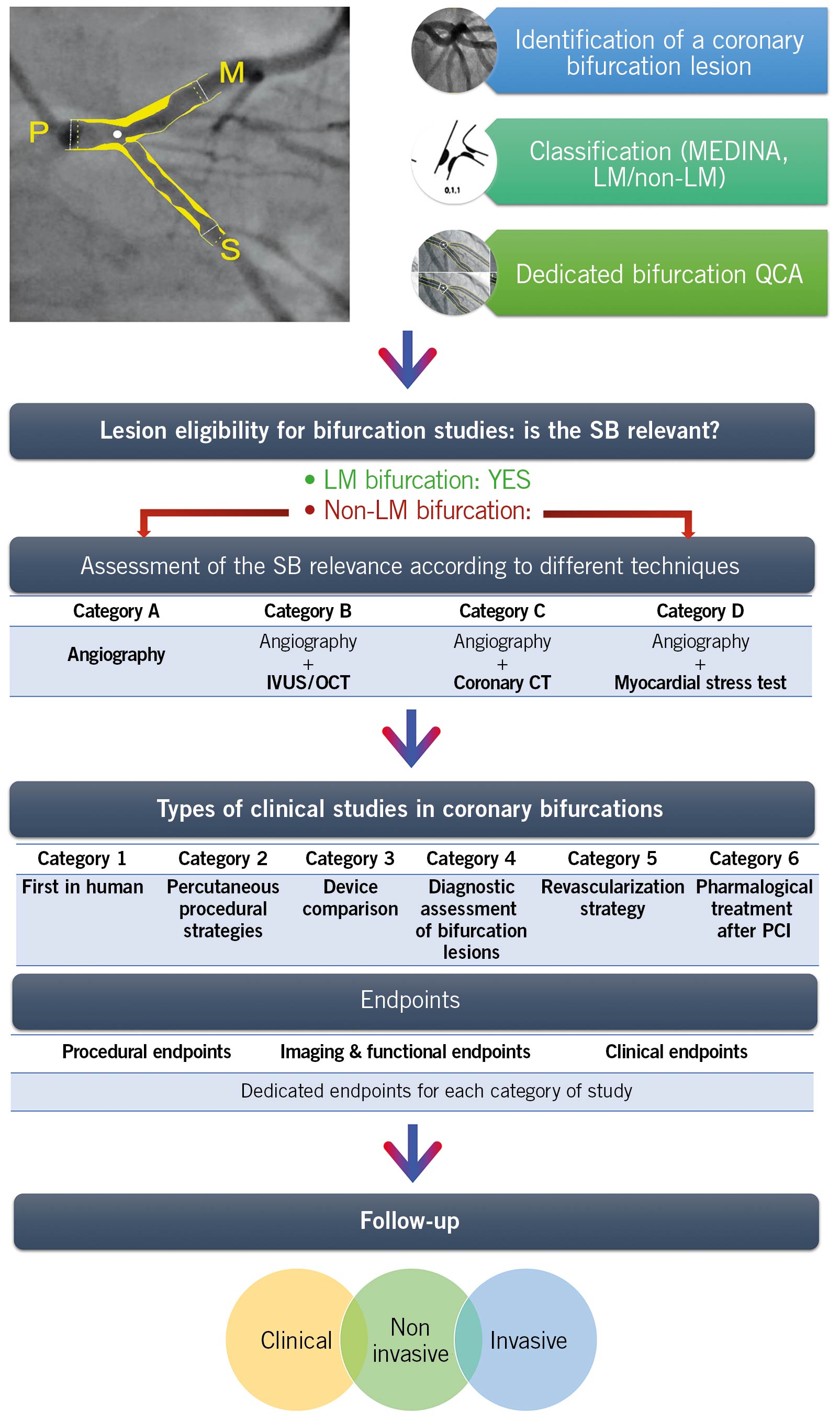
Figure 1. Summary of Bif-ARC Recommendations. After identifying a bifurcation lesion, this should be classified according to MEDINA classification and the left main (LM) involvement. Dedicated bifurcation quantitative coronary analysis (QCA) is strongly advised to be used by sites and core laboratories for quantitative assessment. To evaluate the eligibility of the lesion in a bifurcation trial, its side branch (SB) should be proven to be clinically relevant through different diagnostic techniques according to their availability in the center and the quality and type of the study. The Bifurcation Academic Research Consortium (Bif-ARC) recommends 6 classes of study type, based on the investigation. For every category of study, dedicated endpoints are provided and classified in 3 groups. The follow-up includes clinical, noninvasive, and invasive assessment.
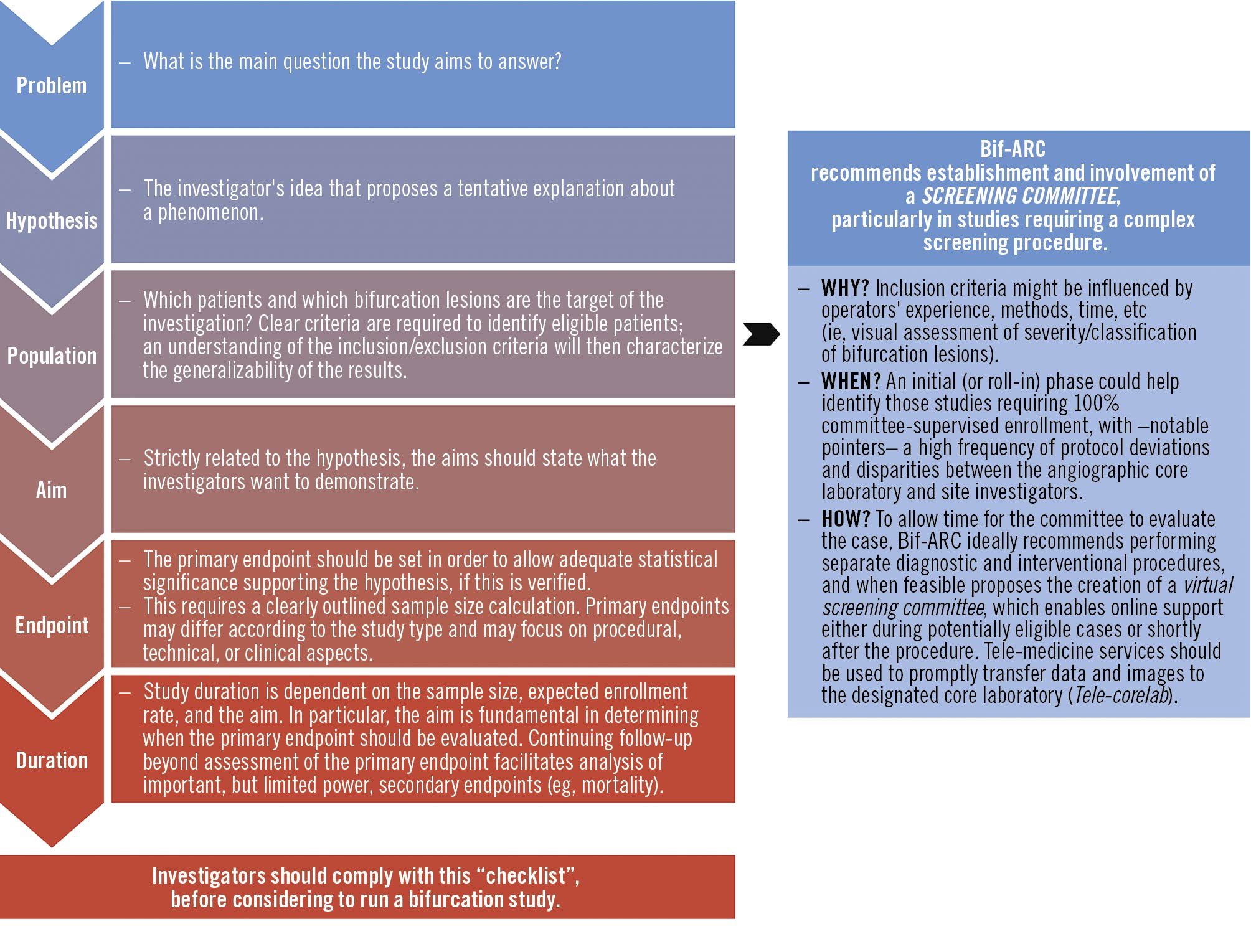
Figure 2. General requirements for coronary bifurcation lesions studies. The investigators should comply with minimal requirements before running a bifurcation study. Bif-ARC: Bifurcation Academic Research Consortium.
1. Anatomical definitions and classification of coronary bifurcation lesions
1.1. DEFINITION
Bifurcation Academic Research Consortium (Bif-ARC) endorses the coronary bifurcation definitions of the last European Bifurcation Club consensus (Table 1, Figure 3).2
Table 1. Anatomical bifurcation and trifurcation lesion definitions.
| Definition | |
|---|---|
| Coronary bifurcation289 | A coronary region consisting of 3 major parts: 1) PMV; 2) DMV (both together forming the MV); and the 3) SB.2 The longest and largest distal branch should be designated the DMV given the linear relationship between diameter, length, flow, and supplied myocardial mass.90 Bifurcation carina is the tissue connecting DMV and SB.Within the bifurcation, we define the POB and the POC (Figure 3).91POB is the center of the largest circle that fits in the bifurcation and touches all 3 contours. The POB is the point where all 3 centerlines (ie, the lines through the middle of the vessel) from the PMV, DMV, and side branch meet (Figure 3).POC represents the smallest possible independent region that behaves differently from a single vessel segment. It is defined on the 2D radiographic image as the area or region that encompasses the start and the end of the bifurcation region. The intersections of the largest circle, touching all 3 contours of the bifurcation, with the centerlines of each vessel indicate the boundaries of the POC (Figure 3).Considering the limitation of 2D angiography, such entities should be identified in the optimal angiographic view for a given bifurcation, which requires no overlap of distal branches, minimal foreshortening, and displaying of the widest bifurcation angle. |
| Bifurcation lesion2 | Angiographically, a bifurcation lesion is defined as a coronary stenosis adjacent to and/or involving an adequate-sized SB (≥2.0 mm in RefD).6 The lesion is considered significant when its %DS is >50 and the MLD in at least 1 of the 3 segments is located ≤4 mm from the POB.689 |
| Relevant side branch92 | Relevance, according to the proposed algorithm (Figure 5), to be considered only if RefD ≥2.0 mm. |
| Coronary trifurcation93 | Anatomically, we define a trifurcation as a division of an MV into 3 branches, each of which has a lumen diameter ≥2.0 mm. The DMV is defined as the longest and the largest branch, likely reflecting the largest perfusion territory. Between the SBs, the one with the larger diameter is defined as SB1, while the other is SB2.93 |
| Trifurcation lesion93 | Trifurcation lesions are defined by a %DS ≥50 within 4 mm from the POB involving either the MV (proximal and/or distal), with or without significant disease in either 1 or both SBs. |
| %DS: percentage diameter stenosis; DMV: distal main vessel; MLD: minimum lumen diameter; MV: main vessel; PMV: proximal main vessel; POB: point of bifurcation; POC: polygon of confluence; RefD: reference diameter; SB: side branch. | |
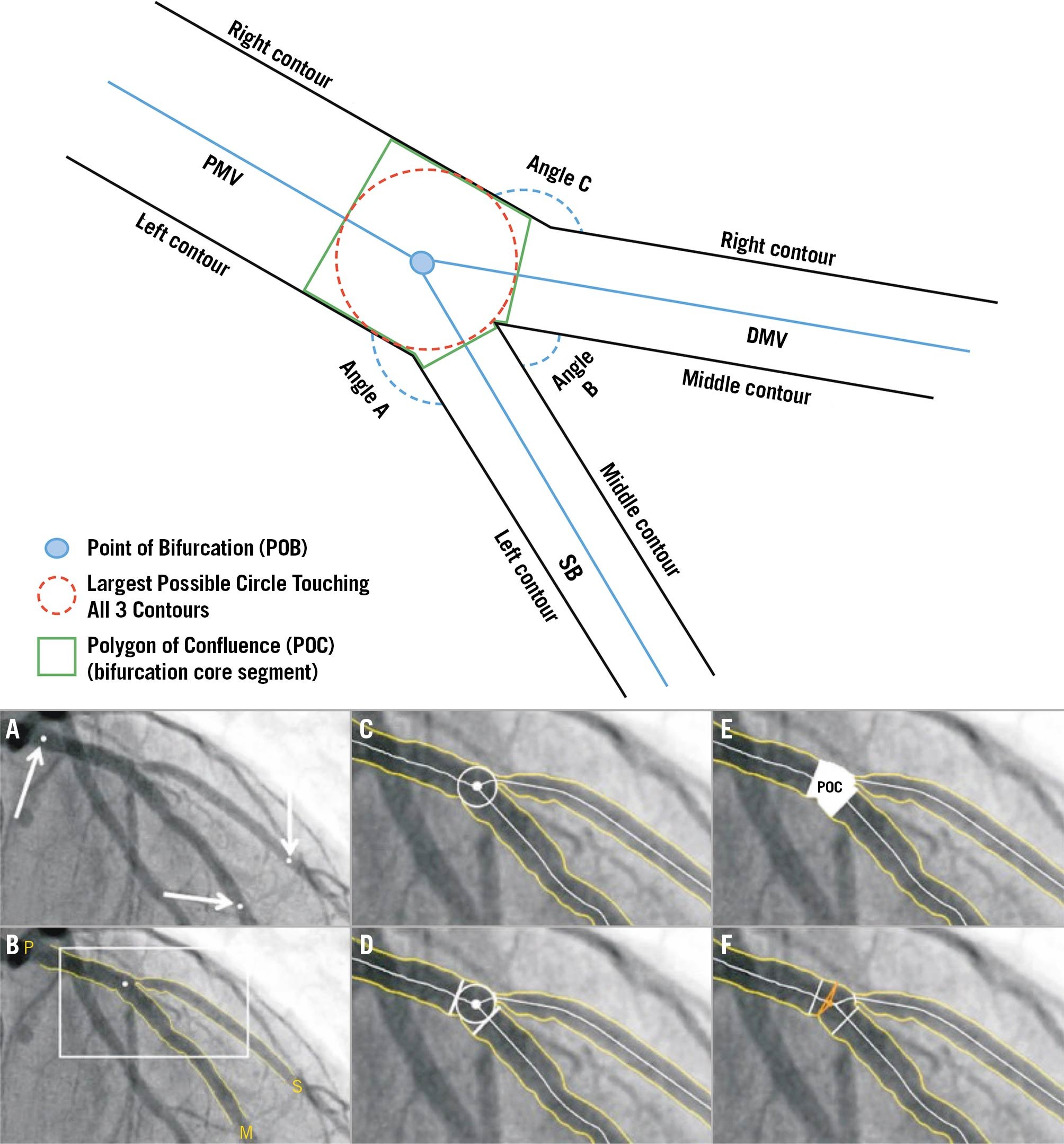
Figure 3. Coronary bifurcation composition. (Top) Schematic representation of coronary bifurcation components. Angle A: access; Angle B: between; Angle C: PM-DM vessel angle. (A to F) Case example of coronary bifurcation on angiography analyzed with dedicated bifurcation quantitative coronary analysis. Arrows: identification of PMV, DMV, and SB by the analyst. DMV: distal main vessel; PMV: proximal main vessel; POB: point of bifurcation; POC: polygon of confluence; SB: side branch.
1.2. CLASSIFICATION
Bif-ARC recommends that dedicated bifurcation trials adopt the MEDINA classification, which has gained acceptance for being both simple and prognostically relevant.34
According to that, we can identify “true” bifurcation lesions, involving a significant (≥50%) diameter stenosis (%DS) both in the main vessel (MV) and SB (ie, MEDINA 1,1,1; 1,0,1; or 0,1,1), and “non-true” lesions in all other cases.5
In the presence of a trifurcation, Bif-ARC recommends using the adapted MEDINA classification (Figure 4). The 4 numbers must be in order of diameters of the distal segments, corresponding, respectively, to the proximal MV (PMV), distal MV (DMV), SB1, and SB2. To avoid confusion, we suggest associating the MEDINA class to the abbreviated name of each segment (ie, MEDINA left main coronary artery [LM], left anterior descending coronary artery [LAD], left circumflex coronary artery, ramus: 0,1,1,0).6
Although site-reported MEDINA can be based on visual assessment alone (somewhat inaccurate7), dedicated bifurcation quantitative coronary analysis (QCA) software should be used either onsite or by core laboratory analysis. To improve onsite assessment, Bif-ARC suggests using intravascular imaging obtained with intravascular ultrasound (IVUS) or optical coherence tomography (OCT),8 which have been reported to be more accurate in detecting atheroma compared with invasive angiography.91011 Notably, intracoronary imaging performed using motorized pullbacks at a set rate permits detailed analysis and is an important parameter of quality because this is the only way to accurately estimate lesion length.
According to the MEDINA classification, Bif-ARC also suggests a ranking of bifurcation lesions in order to evaluate and compare their severity (from highest to lowest severity):
1) 1,1,1; 2) 1,1,0; 3) 1,0,1; 4) 0,1,1; 5) 1,0,0; 6) 0,1,0; and 7) 0,0,1.
Moreover, Bif-ARC recommends classifying the bifurcation lesions in LM and non-LM bifurcations, in addition to SB size and atherosclerotic involvement. SBs with a diameter <2.75 mm are “minor” SBs, whereas those with a diameter ≥2.75 mm are “major” SBs. The length of a SB lesion influences the complexity of SB intervention; as such, a SB lesion ≥10 mm renders the treatment potentially more challenging.12
As per MEDINA classification, the SB size should be evaluated using bifurcation dedicated QCA, or in case of onsite assessment and unavailability of QCA, through intravascular imaging to improve its accuracy (cf. the Intravascular Imaging in Bifurcation Lesions section in the Supplemental Appendix).
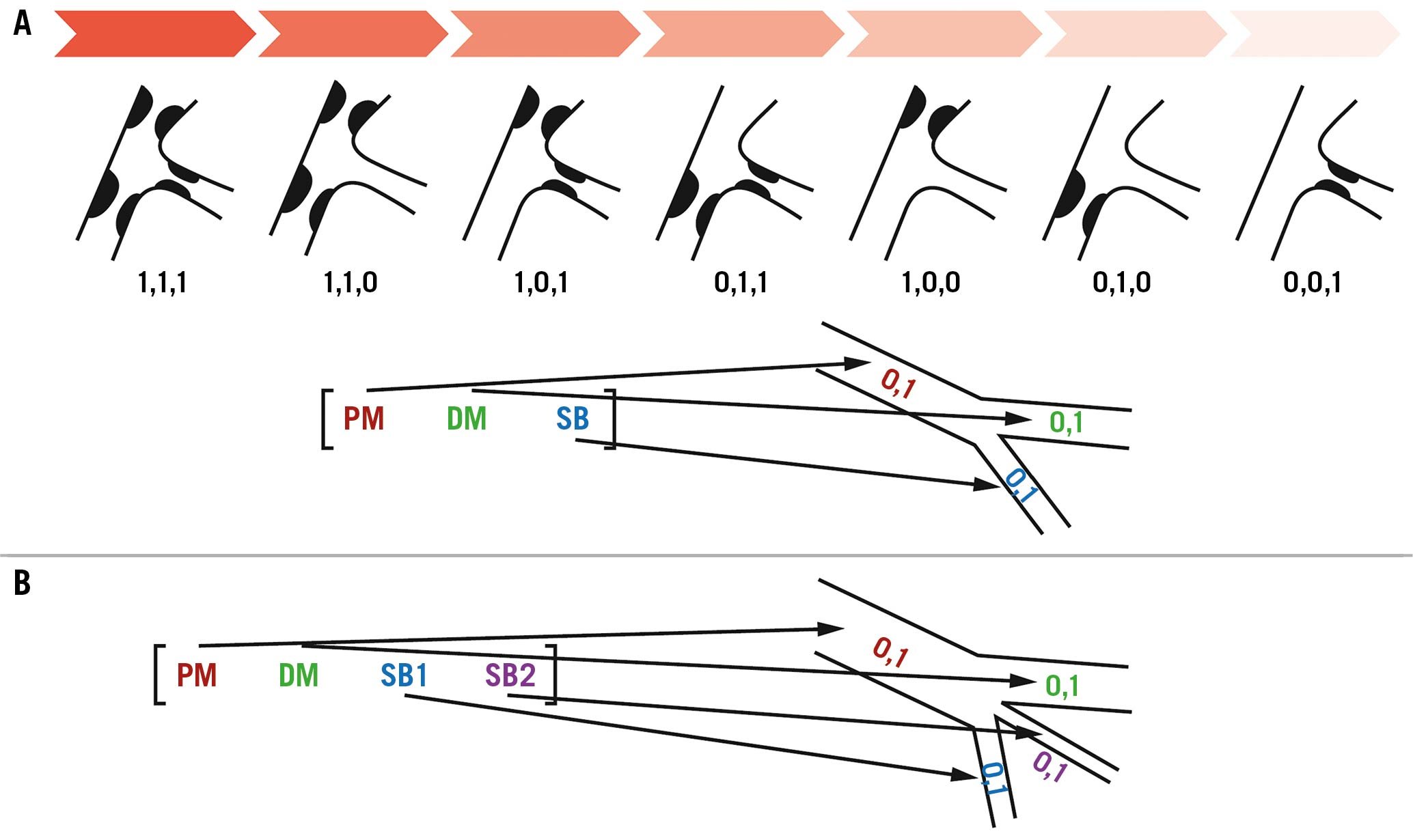
Figure 4. MEDINA classification. Schematic representation of MEDINA classification for bifurcation lesions (A) showing the ranking of lesions severity (from highest to lowest severity), and adapted MEDINA classification for trifurcation lesions (B). Abbreviations as in Figure 3.
1.3. DEDICATED BIFURCATION QCA
The diameters of the 3 segments of a bifurcation lesion follow the Murray’s law (Finet’s and Huo-Kassab formulas).131415 Single-vessel analysis overestimates the reference vessel diameter (RVD) at the ostia of the distal branches, thus overestimating the %DS. By contrast, when the PMV is used for the RVD, the %DS is underestimated. Therefore, the following dedicated 2-dimensional (2D) bifurcation QCA algorithms were developed by incorporating the principles of fractal geometry based on mass conservation (Mandelbrot Set, and fractal object self-similarity) to address the “step-down” reduction in diameter in the bifurcation branches: CAAS bifurcation software (Pie Medical Imaging) and QAngio XA bifurcation software (Medis Medical Imaging Systems) (Figure 5). The accuracy and precision of these packages have been compared in vitro with bifurcation Plexiglas phantoms, and both have proved to be more accurate than single-vessel QCA; therefore Bif-ARC recommends sites and core laboratories use these software for dedicated bifurcation QCA measurements. For further information, see the Technical details and instructions on performing dedicated bifurcation QCA in the Supplemental Appendix and Supplemental Figure 1.
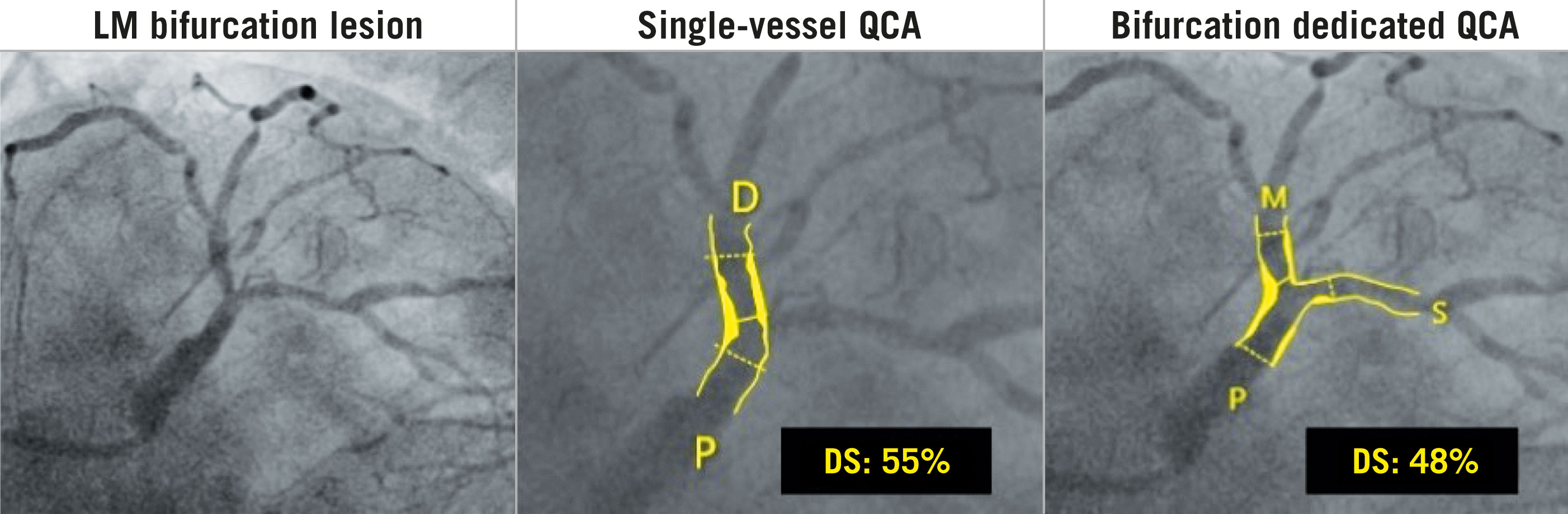
Figure 5. Pseudo-Fractal Geometry and Dedicated QCA. Without implementation of dedicated bifurcation algorithms, taking into account the natural step-down phenomenon of the vessels in presence of bifurcations, the single-vessel QCA leads to erroneous estimation of the main vessel and side branch reference diameters, with over/underestimation of related stenosis. D: distal; DS: diameter stenosis; LM: left main coronary artery; M: main vessel; P: proximal; QCA: quantitative coronary analysis; S: side branch.
2. Target bifurcation lesions for bifurcation studies
2.1. INDICATIONS FOR BIFURCATION LESION REVASCULARIZATION
Both acute and chronic coronary syndromes (ACS and CCS) involving bifurcation lesions deemed suitable for revascularization can be included in bifurcation trials, as per the study design. In CCS with angiographically intermediate stenosis (%DS <70), documenting ischemia is recommended via noninvasive stress testing or invasive functional assessment (with SB assessment limited to MEDINA 0,0,1 lesions), with revascularization indicated in accordance with the latest European and American guidelines on coronary artery revascularization1617 (Supplemental Table 4). In ACS cases, revascularization is guided by the detection of plaque disruption and/or thrombus at the site of the bifurcation, plus physiology.171819
In the presence of multivessel coronary disease, the heart team evaluation should be emphasized by protocol. The selection of the target vessel, the method of revascularization, and the prediction of the patient’s prognosis may be guided by the SYNTAX Score 2020,20 taking into account functional evaluation in its calculation (functional SYNTAX Score), whereby the functional SYNTAX Score is calculated by counting only ischemia-provoking lesions.21
2.2. LESION ELIGIBILITY FOR BIFURCATION STUDIES ACCORDING TO SB PROGNOSTIC RELEVANCE
A significant coronary lesion has 3 potential consequences: 1) symptoms (neurogenic component, subjective22); 2) ischemia (subtended ischemic myocardium, objective); and 3) prognosis (resulting from the amount of myocardium at ischemic or electrical risk).23
The relevance of the SB is fundamental to define the bifurcation lesion as such, and thereby eligibility for inclusion in a dedicated bifurcation study.
Previous clinical studies, mostly underpowered, suggest that RVD could be used as a surrogate marker for the extent of myocardial territory, thereby determining the clinical relevance of that SB.24 However, most studies including only large branches failed to prove the benefit of aggressive intervention for SBs over conservative treatment, underlining the limitation of angiographic vessel size in defining the clinical significance of a branch, or our incorrect understanding of what really constitutes a significant SB.252627 Recent studies using computed tomography (CT) coronary angiography demonstrate alternative methods to assess the myocardial territory subtended by a specific vessel.28 Of note, one study reported that only about 20% of non-LM SBs supply a myocardial mass ≥10% of the left ventricular (LV) mass.2930 However, the supplied myocardial mass is always larger than the ischemic myocardial mass, and the 2 parameters are not interchangeable, nor can they accurately describe electrical and other adverse prognosis.
Previous studies indicate a 10% cutoff in ischemic myocardium is the minimum to justify revascularization over medical therapy (except for a chronic total occlusion), with respect to improved prognosis (cardiac death).23 This cutoff represents an important benchmark combining the ischemic and prognostic implications of a coronary lesion. Although this evidence is not specifically focused on bifurcation lesions, any coronary stenosis causing such a grade of ischemia should be considered relevant, regardless its location in the coronary tree. Therefore, we propose that criterion for identifying a significant SB. Defining SBs on the basis of symptoms (eg, angina) is a much more challenging prospect, whereas development of significant angina after SB compromise clearly indicates its relevance.
Therefore, we propose that a SB should be defined as “relevant” if symptoms are stemming from a large amount (>10%) of ischemic SB-related myocardium, impacting prognosis.
Assuming that SBs of LM bifurcations are always considered prognostically relevant, to specifically estimate or quantify the SB-related myocardium at risk of non-LM bifurcations, Bif-ARC proposes a standardized algorithm based on available diagnostic techniques to help classify bifurcation studies into different categories, which should be defined in the study protocol, before enrolment commences (Figure 6). This strategy will require specific expertise from the centers involved; however, it will facilitate comparisons across studies of similar technical requirements.
The minimum requirement to assess the relevance of a SB is a baseline coronary angiogram in 2 orthogonal views (Category A), which allows indirect measurements (ie, SB length, anatomical scores) that are surrogates of SB-related myocardial mass, and despite their deficiencies, offer an estimation of its relevance. The minimum required criterion is an angiographic reference diameter ≥2 mm, plus additional criteria according to the diagnostic technique. The use of additional imaging (eg, intravascular imaging, CT angiography, nuclear imaging, etc), when available, is strongly recommended to increase the accuracy of the assessment and quality of the study (Categories B, C, and D), and in these cases, it should drive the definition of the SB relevance instead of the angiography.
Among them, we recommend:
– Intravascular imaging (Category B): increased accuracy of SB reference vessel dimension measurements;
– Coronary CT angiography (Category C): target SB length >73 mm,31 or dedicated coronary CT software to calculate SB-related myocardium at risk >10%283132;
– Exercise or pharmacological stress echocardiography (Category D): ≥3 segments involving the SB territory showing stress-induced moderate or severe hypokinesia, or akinesia1633;
– Myocardial perfusion single-photon emission computed tomography (SPECT) (Category D): SB-related ≥10% LV ischemia1633;
– Cardiac magnetic resonance (CMR) perfusion (Category D): ≥2 contiguous reduced perfusion segments involving the SB territory1633;
– Hybrid cardiac imaging (Category D): SPECT/CT, positron emission tomography/CT,34 or CMR/CT, to identify SBs subtending myocardium at risk >10%.
Retrospective analysis of these imaging techniques is also allowed if acquired in the previous 3 months, provided clinical status has remained the same.
In studies providing core lab analysis, the SB relevance, being a critical part of lesion eligibility, should also be evaluated by the core lab, whatever diagnostic test is used.
Angiography is the must-have test, and it is necessary when other techniques are unable to identify SB-related myocardial ischemia (healthy SB or MV/SB ischemic territories not identifiable). For diagonal SBs, we recommend using the SNuH (size, number, highest) score, which is a simple anatomical scoring system based on angiography to estimate the mass of myocardium at risk.35 For further details, see the SB prognostic relevance according to the SNuH score section in the Supplemental Appendix.
Performing intravascular imaging is important considering that angiography can underestimate the exact size of the SB. Direct comparisons between angiography and intravascular imaging reveal a 5% underestimation of the RVD using QCA,9 whereas the most accurate measurement is obtained using OCT.1036 Therefore, Bif-ARC suggests using intravascular imaging, and preferably OCT, to ascertain the reference diameter of the SB. Regardless of the adopted strategy, all image acquisition should be preceded by intracoronary nitroglycerin administration to maximize vessel size.
Integrated techniques involving coronary CT angiography and SPECT or positron emission tomography, by coregistration and fusion of either standalone or combined acquired images, offer incremental diagnostic value beyond that of either imaging modality alone, and in the context of a bifurcation lesion, the integration of dual imaging appears to improve the identification of the culprit vessel and the size of the subtended myocardium.34
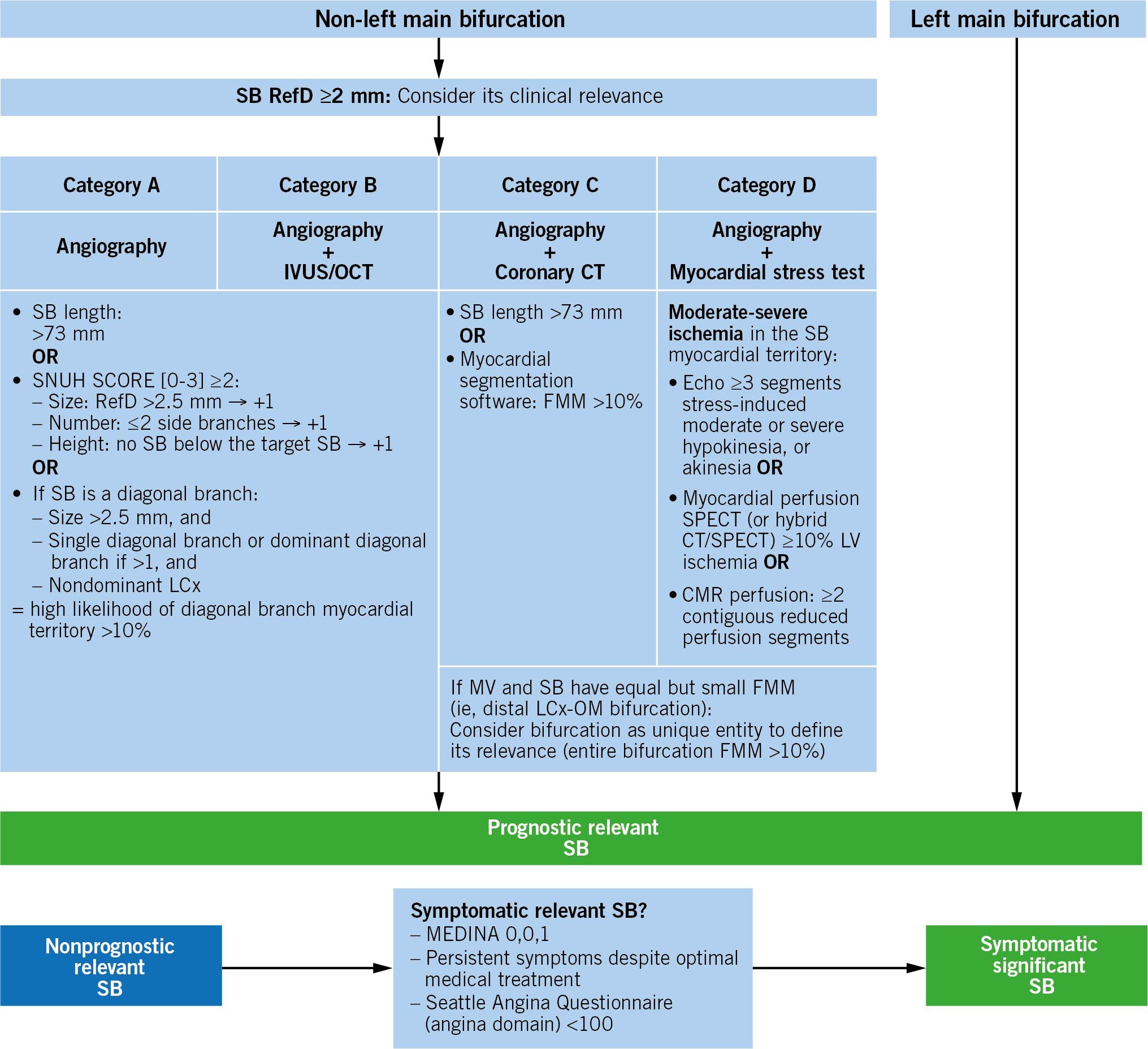
Figure 6. Algorithm to determine the lesion eligibility according to the sb relevance. CMR: cardiac magnetic resonance; CT: computed tomography; FMM: fractional myocardial mass; IVUS: intravascular ultrasound; LCx: left circumflex coronary artery; MV: main vessel; OCT: optical coherence tomography; OM: obtuse marginal branch; RefD: reference diameter; SB: side branch; SPECT: single-photon emission computed tomography
2.3. LESION ELIGIBILITY FOR BIFURCATION STUDIES IN SYMPTOMATIC, BUT NONPROGNOSTICALLY RELEVANT SB LESIONS
Despite the absence of any of the aforementioned criteria of a prognostically relevant SB, the bifurcation lesion may still be considered eligible for a bifurcation study. SBs supplying <10% of the myocardium but still causing symptoms despite optimal medical therapy including aggressive therapy aimed at plaque regression, may require revascularization to maintain quality of life (QoL), even with uncertain prognostic benefit. Indeed, acute occlusion of even nonrelevant SBs can cause clinically evident myocardial infarctions.
Discrimination of SB-related angina symptoms in the presence of significant plaque involving the MV (either proximal or distal) is not possible. The only scenario in which angina symptoms can be unequivocally attributed to a SB is when the bifurcation disease has a MEDINA 0,0,1 pattern. In this case, a bifurcation with a nonrelevant SB can be considered for bifurcation studies if the symptoms are unremitting (Figure 6).
In the absence of symptoms, there are also scenarios when a nonprognostically relevant SB could be considered eligible for a bifurcation study. In patients with poor LV function and chronic total occlusions –not amenable to recanalization– but collateralized by SBs stemming from bifurcation lesions, restoring patency of the SBs may assume an important perfusion role, regardless of size.
MEDINA 0,0,1 lesions without these characteristics do not meet the criteria for bifurcation studies.
2.4. COMPLEXITY OF BIFURCATION LESIONS
Overall, a number of clinical, anatomical, and procedural factors might contribute to the technical difficulties and risk of complications in an individual patient, therefore defining lesion complexity.2
Complex lesions are more likely to have characteristics (eg, long ostial SB lesions) that prompt operators to use longer or multiple stents, associated with higher long-term events,37 however, we do not know whether this aggressive approach is the best way to manage complex lesions. It is therefore imperative to standardize the definition of lesion complexity and trial design in order to make future studies in the context of complex bifurcation lesions comparable.
Their definition can be based on different (or complementary) criteria, according to the method used for evaluation (eg, better calcium distribution evaluation with IVUS than angiography alone). In order to improve comparability between studies addressing complex lesions, Bif-ARC proposes different criteria to define complexity according to the method of evaluation (Table 2).
Table 2. Complexity definition of bifurcation lesions according to diagnostic technique.
| Category | ||
|---|---|---|
| A Angiography |
B Intravascular imaging |
C Coronary CT |
| 1) True bifurcation lesions (MEDINA 1,1,1; 1,0,1; 0,1,1)94+ 1 of the following:• SB disease length ≥10 mm959697• Calcified lesion• Thrombotic lesion• Difficult SB access (higher risk if bifurcation angle A <90°)2) RESOLVE score98Dedicated bifurcation QCA recommended | 1) True bifurcation lesions (MEDINA 1,1,1; 1,0,1; 0,1,1)94+1 of the following:• SB disease length ≥10 mm99100101• Thrombotic lesion• Calcium arc >60° at the culprit lesion site99• Difficult SB access (higher risk if bifurcation angle A <90°)a | 1) True bifurcation lesions (MEDINA 1,1,1; 1,0,1; 0,1,1)94+1 of the following:• SB disease length ≥10 mm99100101• Thrombotic lesion• Calcium arc >60° at the culprit lesion site99• Difficult SB access (higher risk if bifurcation angle A <90° [3D assessment])• Plaque composition: presence of low attenuation plaque in the SB or spotty calcifications within the bifurcation lesion100• Abnormal CT-derived FFR in the SB, suggesting dedicated 2-stent strategy2) CT bifurcation score >1101:• Ca-plaque in PMV (+1)• Low attenuation plaque in PMV/SB (+1)• SB lesion length >5 mm (+1)• MV area/SB area >4.3 (+1)3) CT-derived RESOLVE SCORE102 |
| aAngiography based. Ca: calcium; CT: computed tomography; FFR: fractional flow reserve; QCA: quantitative coronary analysis; other abbreviations as in Table 1. | ||
2.5. INVASIVE FUNCTIONAL ASSESSMENT OF BIFURCATION LESIONS
Either when clinically required to assess ischemia, or when mandated by the study protocol, functional investigation of a bifurcation lesion requires technical precautions, that are fundamental to avoid measurement errors. SB flow is, in normal conditions, less than the flow toward the MV. In the presence of a PMV lesion and additional MV downstream lesions, the flow toward the SB increases (“branch steal effect”), increasing the distal pressure value (Pd) measured by the pressure wire in the SB. As a result, the SB functional assessment may be underestimated.38
Accordingly, we recommend using invasive functional assessment as follows (Supplemental Table 5):
– Before intervention: To evaluate the functional significance of a MV stenosis or pure SB stenosis (MEDINA 0,0,1) when ischemia has not been confirmed elsewhere;
– During intervention: To decide whether additional interventions are required in a jailed SB (ie, occurrence of tight ostial stenosis of SB after crossover stenting of the MV)3940414243;
– After intervention: To assess the functional significance of a jailed SB, or to assess procedural success in the MV and in the SB, if treated. In the first case, a jailed pressure wire was shown utilizable for the assessment.44
Similar considerations, except for a few caveats, are valid for LM bifurcation stenoses (Supplemental Table 5).
Image-based functional assessment is a novel diagnostic modality for functional testing of coronary artery stenoses without using pressure wires and/or the induction of hyperemia.45 Unfortunately, the accuracy of these methods is yet to be validated in bifurcation lesions; therefore, whereas this image-based methodology (possibly retrospective) allows a standardized physiological assessment of every lesion involved in a trial, for the time being, it should not replace standard invasive physiological assessment, which remains the gold standard.
Bif-ARC supports investigational use of image-based fractional flow reserve (FFR) analysis pre-treatment, posttreatment, and during follow-up, especially with algorithms using fractal laws (ie, Murray’s law in quantitative flow ratio46). For further details, see the Image-based functional assessment of bifurcation lesions section in the Supplemental Appendix.
2.6. INTRAVASCULAR IMAGING
Angiography often limits a comprehensive evaluation of bifurcation disease, whereas intracoronary imaging offers better definition of plaque composition (eg, localizing and quantifying calcium and lipid) and better assessment of its extension. In addition, it provides crucial periprocedural information (eg, lesion coverage, wire positions, stent expansion, and strut apposition) to help optimize treatment. Its feasibility during trials, and routine practice, is well documented.474849 Therefore, Bif-ARC supports the use of intravascular imaging as an adjunctive technique in bifurcation trials.
Specific recommendations for optimal image acquisition and core lab analysis are reported in the Intravascular imaging in bifurcation lesions section of the Supplemental Appendix.
3. Types of clinical studies in coronary bifurcations
Any investigation relating to a bifurcation lesion, including new dedicated devices, pharmacological, and/or new surgical or percutaneous treatments, requires a specific study design with standardized endpoints.
As a primary classification, Bif-ARC suggests separate trials of LM and non-LM bifurcations in order to prevent including both types of bifurcation lesion in the same study. In particular cases where the investigators desire to include both types, their inclusion in the study should be stratified according to that variable, or a stratified randomization should be considered.
Beyond this, Bif-ARC proposes the following classification of studies (Table 3):
Table 3. Coronary bifurcation study types and related endpoints.
| Type of study | Description | Procedural endpoints | Imaging and functional endpoints | Clinical endpoints |
|---|---|---|---|---|
| Procedural strategies comparison | Comparison of provisional vs upfront 2-stent strategy or 2 different 2-stent strategies (as per ITT) eg, DK-Crush II trial: DK crush double stenting vs provisional stenting in coronary bifurcation103 | 1) Intended primary strategy success (eg, crossover rate: the placement of a second stent in the SB, as part of a declared provisional strategy in the preprocedural planning, is not considered a crossover to a 2-stent strategy in strategies comparison studies On the contrary, it is, in studies comparing 1- vs 2-stent procedures Procedural strategy reported as per MADS-2)2) Procedural success:• Device success• Free from event during the index hospitalization (CV death, TBR, PMI, any stroke, BARC 3 or 5 bleeding)3) Health-economic endpoints:• Procedural time (min)• Procedural cost• Fluoroscopy time (min)• Contrast medium amount (mL) | 1) Acute endpoints:• Residual stenosis (bifurcation dedicated-QCA, IVUS, OCT)• Dissection• Perforation• SB temporary flow impairment or occlusion• SB loss• MV and SB TIMI flow• Postprocedural invasive functional assessment and/or image-based FFR ≤0.89104• IVUS/OCT: underexpansion, malapposition, accidental crush, double stent layers, stent edge dissection, tissue protrusion (see Supplemental Appendix)• Post-PCI systolic-diastolic bifurcation angle B range <10°1052) Late endpoints:• Late lumen loss or gain (in all the bifurcation segments, using the same method as per postprocedural assessment)• Binary restenosis (in all the bifurcation segments, using the same method as per postprocedural assessment)• Functional deterioration or net gain (invasive or image-based FFR ≤0.89) |
1) BOCE:• CV death• Target bifurcation-related MI• Target bifurcation-related ischemia• TBR2) Efficacy endpoint:• Target vessel revascularization• Target bifurcation-related ischemia• TBR3) Safety endpoint:• BARC 3 or 5• Definite ST• Any stroke• Any MI• CV death• All-cause death |
| Device comparison | Comparison of different devices (DCB vs stent, ie, BABILON trial106: DCB in both branches + BMS in PMV vs DES in MV only; or new dedicated bifurcation devices vs conventional devices, eg, POLBOS II trial: BiOSS LIM bifurcation dedicated stent vs conventional DES56 | 1) Procedural success:• Device success• SB stenting necessity (if provisional strategy)• Free from event during the index hospitalizations (CV death, TBR, PMI, any stroke)2) Health-economic endpoints:• Procedural time (min)• Procedural cost• Fluoroscopy time (min)• Contrast medium amount (mL) | 1) Acute endpoints:• Residual stenosis (bifurcation dedicated-QCA, IVUS, OCT)• Dissection• Perforation• SB temporary flow impairment or occlusion• SB loss• MV and SB TIMI flow• Postprocedural invasive functional assessment and/or image-based FFR ≤0.89104• IVUS/OCT: underexpansion, malapposition, stent edge dissection, tissue protrusion (see Supplemental Appendix)• Post-PCI systolic-diastolic bifurcation angle B range <10°1052) Late endpoints:• Late lumen loss or gain (in all the bifurcation segments, using the same method as per postprocedural assessment)• Binary restenosis (in all the bifurcation segments, using the same method as per postprocedural assessment)• Functional deterioration or net gain (invasive or image-based FFR ≤0.89) |
1) DOCE:• CV death• Device failure-related MI• Device failure-related ischemia• TBR2) Efficacy endpoint:• Target vessel revascularization• Target bifurcation-related ischemia• TBR3) Safety endpoint:• BARC 3 or 5• Definite ST• Any stroke• Any MI• CV death• All-cause death |
| Diagnostic assessment comparison | Comparison between different physiological evaluation (both invasive and noninvasive) (ie, FFR vs NHPR in bifurcation lesions) | 1) Assessment feasibility of the intended bifurcation segment before the treatment2) Safety:• Any complications related to the assessment3) Health-economic endpoints:• Procedural time (min)• Procedural cost• Fluoroscopy time (min)• Contrast medium amount (mL) | 1) Accuracy of the investigated evaluation method (at preprocedure, postprocedure, follow-up)2) Reproducibility | 1) BOCE:• CV death• Target bifurcation-related MI• Target bifurcation-related ischemia• TBR |
| First-in-human studies | Comparison between a new device and historical data or predefined benchmarks (ie, TRYTON trial)107 | 1) Procedural success:• Device success• Free from event during the index hospitalizations (CV death, TBR, PMI, any stroke) | 1) Acute endpoints:• Residual stenosis (bifurcation dedicated-QCA, IVUS, OCT)• Dissection• Perforation• SB temporary flow impairment or occlusion• SB loss• MV and SB TIMI flow• Postprocedural invasive functional assessment and/or image-based FFR ≤0.89104• IVUS/OCT: underexpansion, malapposition, device fracture, stent edge dissection, tissue protrusion (see Supplemental Appendix)• Post-PCI systolic-diastolic bifurcation angle B range <10°1052) Late endpoints:• Late lumen loss or gain (in all the bifurcation segments, using the same method as per postprocedural assessment)• Binary restenosis (in all the bifurcation segments, using the same method as per postprocedural assessment)• Functional deterioration or net gain (invasive or image-based FFR ≤0.89) |
Objective performance criteria (vs historical data or predefined benchmarks)51:1) Safety endpoint:• All-cause death CV death• Any MI• Definite ST2) Efficacy endpoint:• Any coronary revascularization• Target vessel revascularization• TBR3) Composite efficacy and safety:• CV death, target vessel-MI, and TBR (DOCE)• All-cause death, any MI, and any revascularization (POCE) |
| Postprocedural pharmacological comparison | Comparison between different antiplatelet strategies after PCI (DAPT vs SAPT; short DAPT vs long DAPT; ie, GLOBAL LEADERS bifurcation subgroup study)108 | 1) Final strategy adopted (ie, 1-stent vs 2-stent; procedural strategy reported as per MAD-2)2) Procedural success:• Device success• Free from event during the index hospitalizations (CV death, TBR, PMI, any stroke, BARC 3 or 5 bleeding) | 1) Acute endpoints:• Residual stenosis (bifurcation dedicated-QCA)• Dissection• Perforation• SB temporary flow impairment or occlusion• SB loss• MV and SB TIMI flow• Postprocedural invasive functional assessment and/or image-based FFR ≤0.89104• IVUS/OCT: underexpansion, malapposition, stent edge dissection, tissue protrusion (see Supplemental Appendix)• Post-PCI systolic-diastolic bifurcation angle B range <10°1052) Late endpoints:• Late lumen loss or gain (in all the bifurcation segments, using the same method as per postprocedural assessment)• Binary restenosis (in all the bifurcation segments, using the same method as per postprocedural assessment)• Functional deterioration or net gain (invasive or image-based FFR ≤0.89) |
1) Bleeding endpoint:• BARC 3 or 52) POCE:• All-cause death• Any stroke• Any MI• Any revascularization3) NACE• Bleeding endpoint• POCE4) Nonadherence classifications according to NARC745) PROMs (ie, SAQ)81 |
| Revascularization type comparison (percutaneous vs surgical) | Comparison between the 2 revascularization strategies (eg, bifurcation LM subgroups of SYNTAX, EXCEL trials109) | PCI arm1) Procedural success:• Device success• Free from event during the index hospitalizations (CV death, TBR, PMI, any stroke, BARC 3 or 5 bleeding)CABG arm1) Procedural success:• Successful performance of the intended coronary revascularization surgical strategy• Free from event during the index hospitalizations (CV death, TBR, PMI, any stroke, BARC 3-5 bleeding) | 1) Acute endpoints:PCI arm• Residual stenosis (bifurcation dedicated- QCA, IVUS, OCT)• Dissection• Perforation• SB temporary flow impairment or occlusion• SB loss• MV and SB TIMI flow• Postprocedural invasive functional assessment and/or image-based FFR ≤0.89104• IVUS/OCT: underexpansion, malapposition, stent edge dissection, tissue protrusion (see Supplemental Appendix)• Post-PCI systolic-diastolic bifurcation angle B range <10°105• Residual SYNTAX score2) Late endpoints:• Functional deterioration or net gain (invasive or image-based FFR ≤0.89)PCI arm• Late lumen loss or gain (in all the bifurcation segments, using the same method as per postprocedural assessment)• Binary restenosis (in all the bifurcation segments, using the same method as per postprocedural assessment)CABG arm• Graft stenosis >70% or graft occlusion |
1) POCE:• All-cause death• Any stroke• Any repeat revascularization• Any MI2) Bleeding endpoint:PCI arm:BARC 3 or 5CABG arm:BARC 3, 4, or 53) NACE• Bleeding endpoint• POCE4) PROMs (eg, SAQ) |
| BARC: Bleeding Academic Research Consortium; BMS: bare-metal stent; BOCE: bifurcation oriented composite endpoint; CABG: coronary artery bypass graft; CV: cardiovascular; DAPT: double antiplatelet therapy; DCB: drug coated balloon; DES: drug eluting stent; DK-Crush: Double Kissing Crush versus Provisional Stenting for Left Main Distal Bifurcation Lesions; DOCE: device oriented composite endpoint; EXCEL: Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization; ITT: intention-to-treat; IVUS: intravascular ultrasound; MADS: Main-Across-Distal-Side; MI: myocardial infarction; MV: main vessel; NACE: net adverse clinical events; NARC: Non-adherence Academic Research Consortium; NHPR: nonhyperemic pressure ratio; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; PMI: periprocedural myocardial infarction; POCE: patient oriented composite endpoint; POLBOS: POLish Bifurcation Optimal Stenting; PROMs: patient reported outcome measures; SAPT: single antiplatelet therapy; SAQ: Seattle Angina Questionnaire; SB: side branch; ST: stent thrombosis; SYNTAX: Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery; TBR: target bifurcation revascularization; TIMI: Thrombolysis In Myocardial Infarction; other abbreviations as in Tables 1 and 2 | ||||
1. First-in-human studies:
Any study introducing a new device for use in humans for the first time (ie, specifically designed and dedicated to treat bifurcation lesions). The comparison will be made between the new device and historical data, or predefined benchmarks (ie, TRYTON trial).50 The key endpoints of this study type lie in the Objective Performance Criteria.51 To date, multiple devices specifically developed for bifurcation treatment were tested, but often with unsuccessful results, limiting their application.
Unfortunately, most of these were tested in bifurcation lesions with limited clinical relevance, often of small caliber and providing significant interventional challenges. Future dedicated devices should be investigated preclinically and clinically in relevant bifurcation lesions. Therefore, in particular for these studies, Bif-ARC recommends the aforementioned stratification according to the nature of bifurcation: 1) LM bifurcations; and 2) non-LM bifurcations with major or minor SB (RefD ≥ or <2.75, cf. Section 1.2 Classification). To offer the possibility to assess the actual efficacy and safety of new devices, these studies should cover the range of SB sizes applicable by definition, as per device Instructions For Use.
A subgroup of this category consists of technical studies, which are aimed at investigating the feasibility of specific procedural maneuvers, the use of specific procedural tools, or a particular technique to impact on procedural results. Examples include comparing the damage of different types of jailed wire by electronic microscopy,52 or the feasibility of jailing a pressure wire.44
2. Comparison of percutaneous procedural strategies:
Studies comparing different percutaneous techniques to treat a bifurcation lesion belong to this category (eg, DK-Crush [Double Kissing Crush versus Provisional Stenting for Left Main Distal Bifurcation Lesions] and EBC MAIN [The European Bifurcation Club Left Main Study] trials5354). They will encompass different stent strategies (eg, provisional strategy, and the variety of 2-stent strategies including different ways to perform similar techniques [eg, Crush vs DK-Crush), but also any investigation regarding adjunctive mechanical treatment for the bifurcation lesion (eg, the use of plaque modification techniques such as rotational atherectomy, cutting balloons, intracoronary lithotripsy).
Every strategy must be declared prior to the procedure (categorized as intention-to-treat, according to the latest updated MADS (Main-Across-Distal-Side) classification (MADS-2)55 (Figure 7).
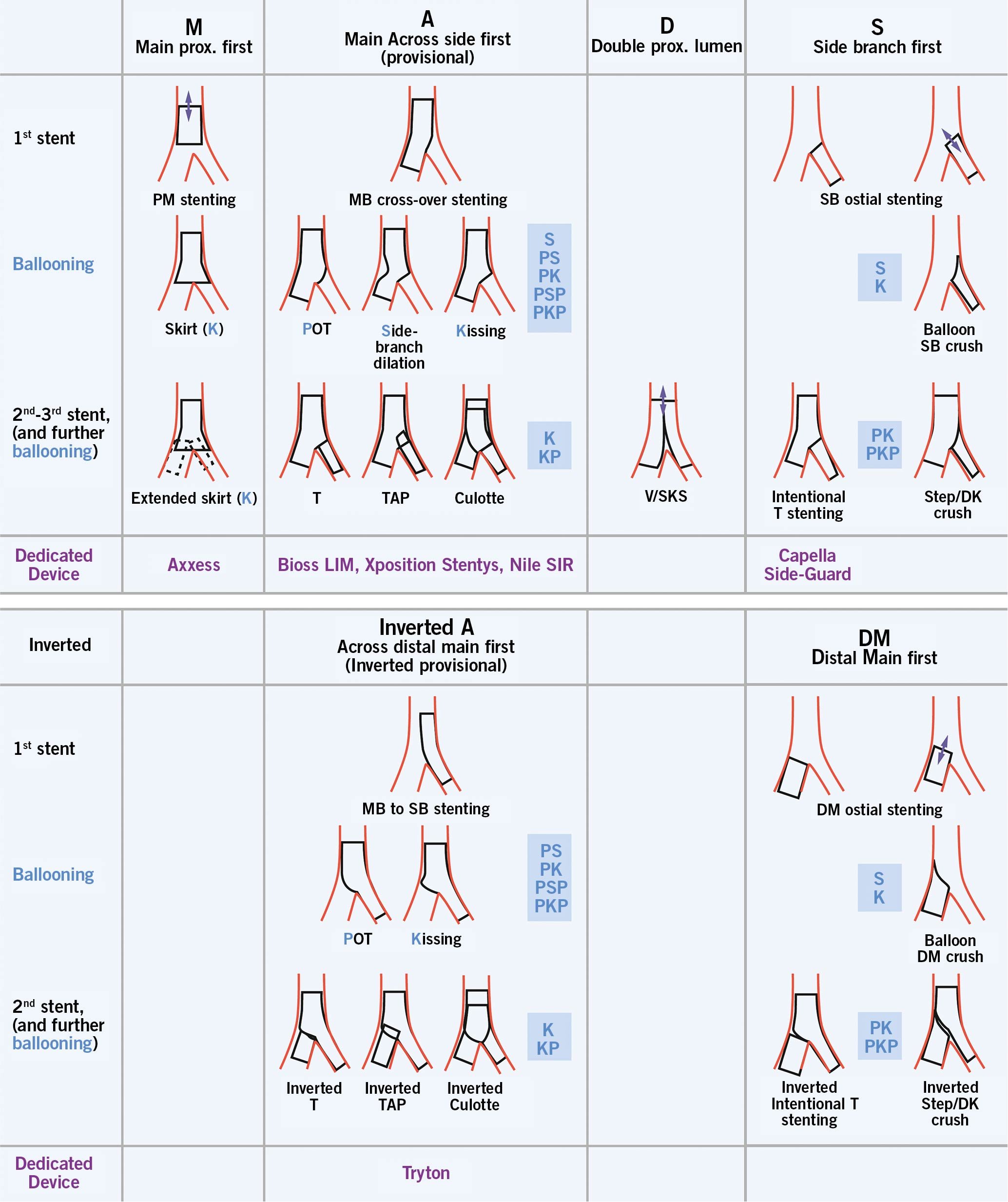
Figure 7. MADS-2 classification of bifurcation stenting techniques. The upper panel shows the standard techniques, whereas the lower panel shows the “inverted” techniques. Blue capital letters refer to the standard ballooning techniques, whereas the lower panel shows the “inverted” techniques. Common combinations of ballooning techniques are described as the sequential blue capital letters. Reproduced from Burzotta et al.55 DK: double kissing; MB: main branch; other abbreviations as in Figure 3
3. Device comparisons:
These studies investigate new or existing devices (both stents and balloons, either dedicated bifurcation or not). The POLBOS II (DES Versus BiOSS LIM) trial56 is an example of this type of study, in which a new dedicated bifurcation stent (BiOSS LIM, Balton) was compared with conventional drug-eluting stents (DES). Of note, this includes both intracategory (ie, DES vs DES) and intercategory device comparisons (eg, DES vs bioresorbable vascular scaffold, DES vs DCB).
4. Diagnostic assessment of bifurcation lesions:
This category includes clinical trials aiming to compare different diagnostic techniques (both imaging and functional). These studies can compare different types of diagnostic imaging (ie, 2D angiography vs 3-dimensional [3D] coronary CT angiography) and different types of physiological assessment (either invasive or image-based). For instance, recent techniques based on 3D reconstruction of a patient’s anatomy and developed to assess flow, shear, and radial stress of a bifurcation lesion, can be included in this group.57
5. Revascularization strategy:
These studies will compare percutaneous vs surgical revascularization treatments and is especially important for LM bifurcation lesions (eg, LM bifurcation subgroup of the SYNTAX [Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery] trial, EXCEL [Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization] trial, etc). The nature of these studies will associate with a greater complexity of disease, and the outcome of LM bifurcation treatment may by influenced by the presence and/or treatment of additional lesions. Any study comparing the 2 strategies in any relevant bifurcation lesion (including non-LM bifurcation) will be part of this group.
6. Pharmacological treatment after percutaneous coronary intervention:
The choice of appropriate antiplatelet regimen, combined or not with anticoagulation, after complex percutaneous coronary intervention (PCI) such as bifurcation PCI, is a particular challenge, with new evidence continuing to emerge.5859 Studies comparing different medical strategies after bifurcation PCI (single vs dual antiplatelet therapy [DAPT], short vs long DAPT, DAPT vs triple therapy, different targets of platelet inhibition, etc) will be included in this category.
Due to the recognized value of bench testing in bifurcation interventions, any novel technique or device should first be tested in bench studies before undertaking a clinical study. Similarly, preclinical studies in large animal models may also reveal important information.
4. Endpoint definitions
For every category of study, Bif-ARC proposes 3 levels of endpoints: procedural, imaging and functional, and clinical (Table 3).
Procedural endpoints include procedure-related outcomes, particularly relevant for studies comparing different techniques or new devices. Additional endpoints regarding the complexity of the procedure (eg, procedural time, x-ray exposure, etc) and health-economic data are also included in this category.
Imaging and Functional endpoints include mechanistic endpoints based on angiographic, intravascular, noninvasive imaging or functional evaluation. These endpoints serve as reports of common angiographic complications, immediate imaging, and function-based results, and are preferably assessed by the core lab. They are applied both post-procedural and at follow-up.
Clinical endpoints encompass efficacy and safety endpoints, and depending on the study, patient or device-related endpoints should be included.
On the basis of the specific study category the consensus defines specific and different composite endpoints. The itemized components, however, should be reported individually. Events should be adjudicated by an independent Clinical Events Committee based on redacted source documents, eventually supported by core lab assessment.
TRIALS AIMING AT PROCEDURAL SUCCESS
With respect to first-in-human studies (category 1), the endpoints are evaluated by comparison with Objective Performance Criteria,51 in particular for those concerning efficacy and safety outcomes. Such studies are not necessarily statistically powered, but stopping rules may be used as criteria of success or failure (ie, ASET [Acetyl Salicylic Elimination Trial] pilot study60). The supervision of the trial by an independent data safety monitoring board, with a consultative role in advising continuation or discontinuation of the trial, is mandatory.
In trials comparing procedural strategies, devices, and in diagnostic assessment studies (categories 2 to 5) procedural and imaging, and functional endpoints have a particular relevance and should be defined as primary endpoints.
Imaging and functional endpoints are required for postprocedural evaluation and at mid-term follow-up (cf. section 6. Follow-Up Methods).
Analysis by an independent core laboratory using standardized operational procedures with predefined analytical plans is strongly recommended. For those studies, where core lab analysis is not available, we recommend following the aforementioned Bif-ARC indications for angiography and intravascular imaging analysis.
Clinical endpoints play a secondary role in these studies, and are suggested to be set as secondary endpoints.
TRIALS INVESTIGATING CLINICAL BENEFIT
In trials focusing on pharmacological regimens, or comparing different types of revascularization strategy (eg, surgical vs percutaneous, categories 6 and 7), clinical composites should be set as primary endpoints, which should include safety (eg, bleeding events) and ischemic endpoints. Net adverse clinical events that incorporate safety-related events and patient-reported outcomes should also be reported.
4.1. INDIVIDUAL ENDPOINTS
All individual endpoints definitions are reported in Table 4 and are outlined in the following text when requiring a bifurcation-specific description.
Table 4. Single endpoints definitions.
| Endpoints definitions | Description |
|---|---|
| Device success | All of:• Successful delivery, balloon expansion, and deployment of the first assigned device, at the intended target lesion/bifurcation. When deployment of >1 assigned device is planned in advance for a single bifurcation lesion (eg, a 2-stent technique), all assigned devices are assessed and reported as 1 device. In that case, only when all assigned devices are successfully implanted at the intended target lesion is this classified as device success.(Multiple attempts using the same instrument are allowed; for example, success at a second attempt with the same [first] investigational device after rewiring the vessel, use of a support catheter, or additional ballooning, vessel preparation, etc).• Successful withdrawal of the device delivery system.• Attainment of a final in-stent or in-scaffold residual stenosis of <30% (or <50% in case of balloon angioplasty alone) with final data reported by core laboratory QCA using dedicated bifurcation software (preferred methodology when no intravascular imaging is provided wsee Supplemental Appendix]) |
| Cardiovascular death | 1. Death caused by acute MI2. Sudden cardiac, including unwitnessed, death3. Death resulting from heart failure4. Death caused by stroke5. Death caused by cardiovascular procedures6. Death resulting from cardiovascular hemorrhage (hemorrhage deriving from cardiac and/or vascular disease/injuries) |
| Periprocedural MI | Evaluation <48 h:• hs-cTn T rise ≥35 URLAND ≥1 of the following criteria:• “Flow-limiting” angiographic complications in a major epicardial vessel (RefD ≥2. 0 mm evaluated by core-lab QCA), at the end of the procedure• New significant Q waves (or equivalent) in 2 contiguous leads, after the procedure• A new wall motion abnormality on echocardiography, after the procedureOR• hs-cTn T rise ≥70 URL(All events should be adjudicated, ideally after core-lab analysis, by an independent CEC) |
| Cardiac biomarkers rise67 | Any CK-MB and/or hs-cTn T rise >6 h after the procedureType 1: due to SB occlusiona) Intraprocedural, after lesion predilationb) Intraprocedural, after device (stent, scaffold) implantationc) Final result at the end of the procedureType 2: due to other angiographic complicationsa) Intraprocedural occlusion of the main branchb) Intraprocedural distal embolizationc) Intraprocedural coronary perforationd) Intraprocedural dissection (after predilation, after device implantation)e) Residual dissection at the end of the proceduref) Intraprocedural thrombusg) Residual thrombus at the end of the procedureType 3: No angiography identifiable causes |
| Stroke | Neuro-ARC definitions (according to ARC-2 criteria) |
| Bleeding | BARC definitions (according to ARC-2 criteria) |
| Target bifurcation-related ischemia | The target bifurcation ischemia is defined in presence of ischemic myocardium supplied by the bifurcation coronary segments treated during the initial procedure. Identification and localization of ischemia requires the use of the same ischemic test, utilized during the inclusion in the study. |
| Target bifurcation revascularization | The target bifurcation lesion is commonly considered as the treated coronary segment during the index procedure plus 5 mm distance from the stent edges or the balloon angioplasty site, applied both for MV and SB in case of bifurcation lesions. When an SB does not undergo either balloon angioplasty or stent placement at the time of the index procedure, but at the time of angiographic follow-up (either mandated or clinically indicated) has developed a stenosis (%DS ≥50 according to bifurcation QCA) Bif-ARC considers that the region extending up to a 5 mm distance from the ostium of the SB should be included within the target bifurcation definition. Target bifurcation revascularization is defined as a repeat percutaneous intervention of the target bifurcation or bypass surgery of the target vessel performed for restenosis or other complication of the target bifurcation.MEDINA classification of the newly diseased bifurcation segments and the repeat revascularized segments is recommended. |
| Target vessel revascularization | The target vessel is defined as the entire major intervened coronary vessel, including side branches.In case of LM-LAD/Circ bifurcation treatment, LM-LAD lesion without significant stenosis in LCx / target vessel: LM-LAD only; otherwise LM, LAD, LCxTarget vessel revascularization is defined as any repeat percutaneous intervention or surgical bypass of any segment of the target vessel including the target bifurcation. |
| Target vessel nontarget bifurcation revascularization | Target vessel nontarget bifurcation revascularization is defined as any repeat percutaneous intervention or surgical bypass of the target vessel for pre-existing disease, disease progression, or other reasons unrelated to the target lesion as defined above. |
| Target bifurcation-related MI | Any MI with angiographic confirmation of culprit lesion corresponding to the target bifurcation previously treatedNonconfirmed bifurcation related MI should be considered as target vessel MI |
| Definite stent thrombosis | Angiographic confirmation: the presence of a thrombus that originates in the stent or in the segment 5 mm proximal or distal to the stent or in the side branch originating from the stented segment and the presence of at least 1 of the following criteria:1) Acute onset of ischemic symptoms at rest2) New electrocardiographic changes suggestive of acute ischemia3) Typical rise and fall in cardiac biomarkers (refer to definition of spontaneous myocardial infarction)OR Pathological confirmation:1) Evidence of recent thrombus within the stent determined at autopsy2) Examination of tissue retrieved following thrombectomy (visual/histology)Early acute: 0-24 h; early subacute: 1 d-30 d; late: 30 d-1 y; very late: >1 y |
| Probable stent thrombosis | Regardless of the time after the index procedure, any myocardial infarction that is related to documented acute ischemia in the territory of the implanted stent without angiographic confirmation of stent thrombosis and in the absence of any other obvious causeEarly acute: 0-24 h; early subacute: 1 d-30 d; late: 30 d-1 y; very late: >1 y |
| SB occlusion | SB flow impairment: SB TIMI flow less than main vessel TIMI flow after procedureSB occlusion: loss of angiographic visualization of SBPMV TIMI flow 0-1: SB flow not assessable |
| MV occlusion | PMV or DMV:1) When TIMI flow grade 3 or 2 at baseline; TIMI flow grade 0 or 1 after the procedure2) When TIMI flow grade 1 at baseline; TIMI flow grade 0 after the procedure3) When TIMI flow grade 0 at baseline and vessel patency (TIMI flow grade 2 or 3) established during procedure; TIMI flow grade 0 after procedure |
| Major dissection (angiographic) | Dissection in the target vessel greater than Type b from National Heart, Lung, and Blood Institute classification110 |
| Perforation | Type 1) extraluminal crater without jet extravasationType 2) pericardial or myocardial blushing without jet extravasationType 3) active jet extravasation exit jet >1 mmType 4) leaking into another cardiovascular cavityType 5) distal perforation |
| Late lumen loss or gain | Difference between the MLD immediately after the procedure and the MLD at follow-up |
| Binary stenosis | >50 %DS at follow-up |
| ARC: Academic Research Consortium; Bif-ARC: Bifurcation Academic Research Consortium; CEC: clinical event committee; CK-MB: creatine kinase-MB; DMV: distal main vessel; hs-cTn: high- sensitivity cardiac troponin; LCx: left circumflex artery; LAD: left anterior descending artery; LM: left main; PMV: proximal main vessel; URL: upper reference limit; other abbreviations as in Tables 1 to 3. | |
DEVICE AND PROCEDURAL SUCCESS
Device success is defined as the composite of successful delivery of the first assigned device at the intended target bifurcation, successful withdrawal of its delivery system, and a final in-stent/scaffold %DS <20 in each stented segment of the bifurcation by visual assessment or <30% by bifurcation QCA61 (<50% in case of balloon angioplasty alone62). When use of intravascular imaging is mandated by the study protocol, device success is defined by a final minimum stent area >80% of the reference vessel area in each stented segment of the bifurcation. The use of bail-out devices (as allocated by randomization) due to edge dissections or geographic miss is not regarded as a device failure but rather as a clinical issue.
Procedural success herein defined as the composite of device success plus additional criteria related to clinical outcomes of the procedure, regardless of whether the protocol-assigned device is used (Table 4).
MYOCARDIAL INFARCTION
Periprocedural myocardial infarction
Myocardial infarction (MI) may occur in the periprocedural period, or long after the procedure because of spontaneous events or late complications related to the investigated device/strategy. The definition of MI, and in particular periprocedural MI (PMI), varies across trials and cardiac societies, and may require different criteria according to study type in order to effectively use the sensitive biomarkers of subtle myocardial injury, and balance them against clearly adverse clinical outcomes.63 Unfortunately, evidence regarding this is scarce; however, it has been shown that PMIs defined by the Society for Cardiovascular Angiography and Interventions (SCAI) criteria, as opposed to the 4th Universal Definition of Myocardial Infarction (UDMI) or the SYNTAX definition, have the best correlation to adverse outcomes after stenting true bifurcations.64
Furthermore, there are still debates about the most accurate cardiac biomarker to use, although in recent times the cardiology community has seen the extinction of creatine kinase-myocardial band (CK-MB) in favor of high-sensitivity cardiac troponin (hs-cTn). Nevertheless, the correlation among the many available type I hs-cTn assays is unclear and leaves room for potential differences between studies or incorrect endpoint definitions.
Considering this, Bif-ARC proposes a modified version of the ARC-2 PMI criteria, incorporating type T hs-cTn,65 currently measured with a single assay. Accordingly, in bifurcation studies, a PMI is defined by either an absolute rise ≥35 upper limit of normal (ULN) threshold for type T hs-cTn plus clinical evidence of MI or an absolute cTn rise ≥70 ULN as a stand-alone criterion within 48 hours of the PCI or coronary artery bypass graft (CABG) (Table 4).
Such criteria reflect the SCAI definition, except for the use of hs-cTn instead of CK-MB, given concerns related to its unavailability. However, the proposed thresholds for hs-cTn have been calculated based on the SCAI CK-MB cutoff values (≥5 ULN and ≥10 ULN, respectively).66
In cases where different cardiac enzymes are measured (ie, cTn and CK-MB), Bif-ARC suggests recording the rate of availability of the different enzymes within the study (ie, % of patients having CK-MB reported).
Given the complexity of the definition of PMI and numerous related issues raised in previous studies, ARC is working to release a PMI-dedicated document in 2022, and this consensus will be updated accordingly.
Spontaneous MI
Bif-ARC endorses the definition of spontaneous MI as per the 4th UDMI (Type 1, 2, 3, 4b, or 4c). Of note, in the 4th UDMI, “prior or silent/unrecognized MI” is defined as abnormal Q waves with or without symptoms in the absence of nonischemic causes, imaging evidence of loss of viable myocardium in a pattern consistent with ischemic etiology, or pathoanatomical findings of a prior MI. Bif-ARC suggests that MI as a component of the primary endpoint does not include “prior or silent/ unrecognized MI” because there is no evidence of cardiac biomarker elevation.
In the setting of bifurcation treatments, Bif-ARC defines target bifurcation-related MI as any MI with angiographic confirmation that the culprit lesion corresponds to the previously treated target bifurcation. Any MI not clearly attributed to a nontarget bifurcation lesion should be considered a target bifurcation-related MI.
The reporting of target bifurcation-related MI is of particular relevance for device-related endpoints.
POSTPROCEDURAL CARDIAC BIOMARKERS RISE
Even when not meeting the criteria for a PMI, Bif-ARC suggests reporting any postprocedural rise in cardiac biomarkers after a minimum of 6 hours from the end of the procedure.67 In the context of investigating a new device, even events of little clinical relevance may be important for the safety profile of the new device. In this setting, when available, CK-MB represents a better biomarker to detect the subsequent fall, given its shorter elimination kinetics.
The enzymatic rise should be classified according to the angiographic findings, as proposed in Table 4 (eg, SB occlusion). The proposed classification discriminates between angiographic complications occurring during the procedure (eg, intraprocedural stent thrombosis or transient SB occlusion) and those complications persisting at the end of the procedure. The record of intraprocedural complications, which may be transient, is relevant considering solid evidence whereby intraprocedural (transient) stent thrombosis has been associated with adverse short-term ischemic outcomes in patients undergoing PCI.68
BLEEDING
Bleeding events should be classified and reported according to the Bleeding Academic Research Consortium criteria.69
International guidelines encourage weighing bleeding risk before selecting a treatment regimen in patients at high risk of bleeding and/or undergoing complex PCI procedures, such as bifurcation revascularization.
Bif-ARC categorizes patients into 2 groups, according to the need for anticoagulant therapy:
1. For patients not requiring anticoagulants, Bif-ARC recommends the recent algorithm proposed by the European Bifurcation Club that bases the decision of DAPT duration on clinical presentation (ACS vs CCS), bleeding risk (high vs low), and use of intravascular imaging.70
2. For patients on anticoagulants (eg, atrial fibrillation), several different antiplatelet/anticoagulant regimens have recently been proposed specifically for patients with atrial fibrillation undergoing PCI, considering the different weight of their bleeding and thrombotic risk.7172
Overall, Bif-ARC supports the use of the ARC-HBR (Academic Research Consortium High Bleeding Risk) tool to evaluate patients’ bleeding risk and in particular their ischemia/bleeding tradeoff, although further validation in the specific context such as bifurcation PCI is needed.73
In addition, for studies investigating pharmacological treatment after bifurcation revascularization, Bif-ARC suggests the collection and analysis of medication adherence according to the 4 classes (Type 0, 1, 2, and 3) proposed by the Nonadherence Academic Research Consortium. The adoption of such classification will afford robustness and consistency in the comparative safety and effectiveness evaluation of investigational pharmacological regimens,74 and frequently requires a per-protocol analysis (cf. section 7. Statistical Consideration).
REPEAT REVASCULARIZATIONS
Nomenclature
Repeat revascularization will be defined according to the vessel/lesion treated, identifying them as target or nontarget, based on the initial site of revascularization.
A target bifurcation revascularization (TBR) is defined as a repeat revascularization of the target bifurcation by PCI or bypass surgery of the target vessel(s), performed for restenosis or another complication of the target bifurcation.
Because some revascularization techniques lead to iterative restenosis–retreatment, Bif-ARC recommends collecting the number of additional repeat revascularizations that can be considered with dedicated statistical approaches (win ratio analysis, Cox-based models for recurrent events, and weighted composite endpoint [WCE] analysis, cf. section 7.2 Statistical Analysis Including Repeated Events and Sample Size Calculation), whereas the first recurrence occurring after the initial treatment is classically included in the time-to-first event analysis.
The target bifurcation is commonly considered as the bifurcation coronary segment treated during the index procedure plus 5 mm from the stent edges or the site of balloon angioplasty, applied to both the MV and SB. When a SB that did not undergo either balloon angioplasty or stent placement at the time of the index procedure, but at the time of angiographic follow-up (either mandated or clinically indicated) has developed a stenosis (%DS ≥50, according to bifurcation QCA), Bif-ARC considers that the region extending up to 5 mm from the ostium of the SB should be included within the target bifurcation definition.
Bif-ARC proposes a new nomenclature, according to which any TBR should be accompanied by the identification of the diseased bifurcation segments using the MEDINA classification (MEDINArestenosis), based on the core lab–dedicated bifurcation QCA75 (Supplemental Table 6).
In trials comparing CABG and PCI, in the CABG arm, the ascertainment of TBR should consider the patency (stenosis or occlusion) of the graft either on the DMV, SB or both branches as a surrogate for restenosis in the native vessels. In such cases, to define the diseased segments, Bif-ARC proposes a modified version of the MEDINA classification consisting of 2 binary values (0 or 1) referring to the grafts towards the DMV and the SB, respectively, preceded by an “x” (representing the PMV = not applicable in case of grafts) (Supplemental Table 7).
Once repeat revascularization is performed, the operators should report the type of revascularization and nomenclature of revascularized segments using the MEDINA classification as shown in Supplemental Table 8 (MEDINArevasc-CABG or MEDINArevasc-PCI).
Target vessel non-TBR is defined as any repeat percutaneous intervention or surgical bypass of the target vessel for pre-existing disease, disease progression, or other reasons unrelated to the target bifurcation lesion as defined in the preceding text.
Adjudication criteria for TBR
Adjudication of repeat revascularization requires clinical, angiographic, and functional criteria.76
A core lab using dedicated QCA bifurcation software is recommended, especially when functional evaluation is not available or provided. Bif-ARC underscores the importance of functional assessment in order to justify the need for repeat revascularization procedures. When the functional test is negative (ie, FFR>0.80) despite the presence of angina pectoris, Bif-ARC suggests investigating the presence of microvasculature dysfunction, from functional or structural origin.77 Supplemental Table 9 reports the hierarchical order of functional and angiographic criteria recommended for event adjudication of clinically indicated repeat revascularizations. The functional assessment of a bifurcation lesion requires precautions as outlined in section 2.5 and in the Supplemental Appendix.
Whenever the functional evaluation of the target lesion is not possible or reliable, and in the presence of a bifurcation QCA %DS ≥50, we recommend categorizing the revascularization as clinically driven, based on either recurrent symptoms or a positive noninvasive ischemia test. A bifurcation QCA %DS ≥70 in the absence of such criteria may also be considered as a clinically indicated revascularization. Of note, any planned staged procedure is not considered a TBR, at least within the protocol-defined timeframe allocated for staged procedures.
5. Procedural, technical, and clinical information to collect
5.1. PROCEDURAL AND TECHNICAL DATA
Procedural and technical data before and after the procedure, and at follow-up should be collected according to the study type. A list of the essential variables that Bif-ARC recommends to be recorded in the case report form are presented in Supplemental Table 10.
Specifically for LM bifurcation studies, given the importance of operators’ experience for clinical outcomes, Bif-ARC recommends reporting the volume of LM bifurcation PCI/year of the center.165378
5.2. CLINICAL DATA
Similarly, clinical variables should be collected at baseline, during the hospital stay, and at the various follow-up visits, according to the type of study. Some differences in data collection are expected for CABG arms in revascularization type comparison trials. The list of essential data to be reported in the case report form recommended by Bif-ARC is detailed in Supplemental Table 11.
6. Follow-up methods
Bif-ARC recommends carrying out follow-up on a 3-level basis:
1. Clinical and patient level (eg, clinical and patient-reported endpoints);
2. Noninvasive testing (eg, ischemia tests, coronary CT);
3. Invasive testing (eg, coronary angiography).
6.1. CLINICAL AND PATIENT-BASED FOLLOW-UP
Bif-ARC recommends the use of composite clinical endpoints at every follow-up visit, as defined for every study in Table 3.
Timing for their evaluation is according to the study protocol, but as a minimum we recommend 12-month clinical follow-up when no angiographic follow-up is required. In order to avoid interference by confounding angiographic findings (eg, restenosis leading to repeat intervention, not clinically indicated), when angiography is mandated by the protocol, clinical endpoints should be collected before invasive follow-up takes place.
In studies comparing surgical vs percutaneous revascularizations, Bif-ARC recommends extending clinical follow-up to 10 years.
The final goal of coronary revascularization, however, is not only to prevent hard cardiac events, but also to improve symptoms, functional status, and the patient’s QoL. From a broad perspective, quality adjusted life-years is the ultimate endpoint for the trialists and the patient, because it represents the combination of survival and QoL gain.
Therefore, Bif-ARC recommends analyzing patient-related outcome measures during follow-up.7980 In the context of bifurcation studies, patient-related outcome measures play a key role in particular in studies investigating the clinical benefit derived from different revascularization treatments (PCI vs CABG) and different pharmacological regimens, when the 2 competing strategies might lead to significant differences in health status as perceived by the patient.
On the contrary, studies investigating bifurcation percutaneous strategies (eg, provisional vs upfront 2-stent strategy) or dedicated bifurcation devices (eg, bifurcated stents vs standard stents), are less likely to produce measurable differences in patients’ perception.
As an assessment tool, it is important to choose the one that best quantifies the domain of health most likely affected by the treatment under investigation. For instance, studies comparing surgical vs percutaneous treatment should use an angina status questionnaire (eg, Seattle Angina Questionnaire)8182 or health status questionnaires addressing in a wider way the impact of the 2 different clinical interventions (eg, Short Form 36 Health Survey Questionnaire).83 Nevertheless, any tool selected by the investigators should have psychometric properties (validity, reliability, responsiveness, and interpretability) proven to measure the intended domain (eg, Seattle Angina Questionnaire, Short Form 36 Health Survey Questionnaire).8183
6.2. NONINVASIVE FOLLOW-UP
Noninvasive testing should be undertaken whenever a clinical suspicion of recurrent ischemia exists, or if mandated by the study protocol. In first-in-human studies, or in the presence of clear ongoing ischemia (eg, unstable angina), however, invasive assessment should be performed first.
The test type is left to trial designers’ discretion (eg, cycle ergometer stress testing, echocardiography stress test, nuclear imaging test, stress CMR, et al).
6.3. INVASIVE FOLLOW-UP
Invasive follow-up consists of a coronary angiogram either as protocol mandated or secondary to adverse events requiring invasive diagnosis/intervention.
It is vital for all studies investigating new devices (first-in-human or through comparison with existing devices) to define their mechanical efficacy, and it is also of relevant benefit in studies comparing percutaneous strategies.
In cases of invasive follow-up, core lab analysis is recommended. In regard to QCA, the same segmental analysis used at the time of postintervention should be considered at this stage. This should include dimensional analysis of residual stenosis and the precise location of treatment failure or restenosis at follow-up (cf. Repeat Revascularization in Section 5.1).
Functional assessment of the target bifurcation is advised to measure its “functional deterioration,” defined as a reduction in the functional values compared with the postprocedural values.
Depending on study design and the interrogated device or strategy, angiography may require intravascular imaging. When required by the study protocol or for clinical reasons, IVUS or OCT can be used according to the aforementioned indications/ criteria.
7. Statistical consideration
The general recommendation of Bif-ARC is to design separate dedicated bifurcation studies for LM and non-LM bifurcations; however, there may be cases when investigators include both in the same study. In this scenario, Bif-ARC recommends stratifying the analysis accordingly and, when randomization is required, mandating a stratification randomization variable.
7.1. INTENTION-TO-TREAT VS PER-PROTOCOL VS AS-TREATED
In bifurcation studies comparing interventional treatments, the rate of cross-over is expected to be higher than others studies, given the complexity of the intervention and the difficulties in predicting results, particularly in the SB. Hence, a clear definition of the operator’s strategy upfront is mandatory, as well as the exact report of the actual strategy/technique used. Primary analysis should be based on the intention-to-treat principle, but Bif-ARC also recommends performing statistical analyses according to the per-protocol and as-treated principles (Supplemental Table 12).
7.2. STATISTICAL ANALYSIS INCLUDING REPEATED EVENTS AND SAMPLE SIZE CALCULATION
The time-to-first-event analysis, which treats all components of the composite endpoint as having equal severity, is the standard method and should be used in the primary analysis.84 On the other hand, this analysis only considers the first endpoint encountered in time. Thus, nonfatal events that occurred earlier have more impact than more serious events such as stroke or death that occur later. Bifurcation lesion revascularization is one of the most complex coronary interventions, and some clinical events, such as MI and repeat revascularization, often occur repeatedly. Several methods have been proposed to overcome these limitations. These methods consider all events occurring during follow-up and/or incorporate the severity of clinical events. They include win ratio analysis, Cox-based models for recurrent events, and WCE analysis (further details in Supplemental Table 13 and in the Statistical analysis including repeated events section in the Supplemental Appendix).
Although all methods have strengths and weaknesses, they may enhance our understanding when components of composite endpoints vary substantially in severity and timing.
Therefore, Bif-ARC recommends their use as pre-specified secondary analyses, according to patient type, devices and strategies used, and events.
For every prospective study, a statistical plan and sample size calculation are mandatory.
In the statistical analysis plan, the method of counting repeated events (Cox-based models for recurrent events and WCE analyses) and the ranking and/or weighting of cardiovascular events (win ratio and WCE analyses) should be prespecified to avoid any uncertainty.85
The sample size calculation should be based on the primary analysis; to date, the time-to-first-event analysis is recommended. When analyses considering recurrent events and/or event severity are used, simulation techniques and dedicated codes are required for sample size calculations.868788
Impact on daily practice
There is a paucity of standardization and comparability across studies involving coronary bifurcation lesions. This document provides standardized definitions and criteria for use in studies of such lesions, from diagnosis through follow-up. Implementation of these recommendations in clinical trials will improve their relevance and improve the quality of care for patients with bifurcation coronary artery disease.
Acknowledgements
The scientific value of the Bif-ARC consensus document has been affirmed by the Society for Cardiovascular Angiography and Interventions.
Conflict of interest statement
This work is supported by a Science Foundation Ireland Research Professorship Award (RSF 1413) including grants to Drs Lunardi and Wijns. Dr Lefèvre has received speaker fees from Abbott, Medtronic and Terumo. Dr Burzotta has received speaker fees from Medtronic, Abiomed, and Abbott. Dr Lassen has received speaker fees from Medtronic, Boston Scientific, Biotronik, Abbott and Biosensors. Dr Darremont has received speaker fees from Edwards. Dr Holm has received institutional research grants and speaker fees from St. Jude Medical and Terumo. -Dr -Johnson has received speaker fees from Abbott, Boston Scientific, Medtronic, and Terumo; and has received institutional funding for fellowships from Boston Scientific and Terumo. Dr Pan has received speaker fees from Abbott, Terumo, and Volcano. Dr -Chatzizisis has received speaker fees and consultation fees from Boston Scientific; and has received research support from Boston Scientific and Medtronic. Dr Banning has received institutional funding of a fellowship from Boston Scientific; and has received speaker fees from -Boston, Abbott, Medtronic, Philips/Volcano, and Miracor. Dr Chieffo has received speaker fees from Abiomed and GADA. Dr Dudek has received grants and personal fees from Boston Scientific, Philips, Abbott, Medtronic, and -Biotronik. Dr Hildick-Smith has received advisory board, consultancy, and research funding from Terumo, Medtronic, Abbott, and Boston Scientific. Dr Dangas has received lecture fees from Bayer and Daiichi--Sankyo; has received institutional and grant support from Daiichi-Sankyo; and has held equity in Medtronic. Dr Stone has received lecture fees from Terumo and Amaranth; has received consulting fees from Shockwave Medical, TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Matrizyme, Miracor Medical, Neovasc, V-Wave, Abiomed, Claret Medical, Sirtex Medical, MAIA Pharmaceuticals, VALFIX Medical, -SpectraWAVE, Ancora, and Vectorious Medical Technologies; holds equity in VALFIX Medical, Ancora, Qool Therapeutics, Orchestra BioMed, Cagent Vascular, Applied Therapeutics, -Biostar, MedFocus, Aria CV, Cardiac Success, and SpectraWAVE; holds stock options in Ancora, Qool Therapeutics, Orchestra BioMed, Cagent Vascular, Applied Therapeutics, Biostar, MedFocus, Aria CV, and Cardiac Success; and has received personal fees from Qool Therapeutics and Orchestra BioMed. Dr Cutlip has received grant support from Cordis; has received travel reimbursement from Abbott Vascular; and has received additional funding for the Clinical Events Committee from Boston Scientific. Dr Mehran has received institutional grant support from Abbott Laboratories, -AstraZeneca, Bayer, CSL Behring, Daiichi-Sankyo, Medtronic, Novartis, -Bristol Myers Squibb, and OrbusNeich; has received consulting fees from Abbott Laboratories (paid to her institution), -Spectranetics (Philips Volcano) (paid to her institution), Boston Scientific, -Medscape (WebMD), -Siemens Medical Solutions, -Roivant Services, Sanofi, Regeneron, and Janssen Scientific Affairs; has received lecture fees from Abbott Laboratories (paid to her institution), and Medtelligence (Janssen Scientific Affairs); has served on advisory boards for Bristol Myers Squibb (fees paid to her institution), PLx Opco, and Medtelligence (-Janssen Scientific Affairs); has served on a data and safety monitoring board (fees paid to her institution) for Watermark Research Partners; has received nonfinancial support from Regeneron; and holds equity in Claret Medical and Elixir Medical. Dr Wijns has received institutional research grants from Terumo, MiCell, and MicroPort; has received honoraria from MicroPort; has been a medical advisor of Rede Optimus Research; and is cofounder of Argonauts, an innovation accelerator. Dr Serruys has received personal fees from Philips/Volcano, SMT, Novartis, Xeltis, and Merillife. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.

