Abstract
Aims: We report the acute and 30 day results from the Tryton I first-in-man (FIM) study, a multicentre prospective single arm study evaluating the safety and feasibility of the Tryton Side-Branch StentTM when used in conjunction with standard drug eluting stent to treat de novo bifurcation lesions within the coronary vasculature.
Methods and results: Clinically, stable patients with de novo coronary bifurcation lesions with side branch diameters 2.25-2.75 mm were treated. Clinical follow-up at 30 days, 9 months and one year is mandated in all patients. Angiographic and IVUS evaluation is performed at the completion of the case and at 6 months. A total of 30 patients (66.0±11.2 years old, 63% male) were treated. Approximately 76% of the treated lesions involved a left anterior descending-diagonal (LAD-Diag), with the remainder of lesions in the left circumflex-obtuse marginal (21%) and right coronary-posterior descending (4%) coronary arteries. Baseline angiographic disease in both main vessel (MV) and side branch (SB) was noted in 50% of patients. In 47% of patients, angiographic disease was limited to the MV, and in one patient (3%) to the SB ostium. Two major major adverse cardiac events (MACE) were noted (94% success Rate). All patients were treated via 6 Fr guides. Angiographic success including final kissing balloon inflation was achieved in all patients in whom the Tryton StentTM could be tracked to the lesion site (97% angiographic success). No additional MACE events were noted between hospital discharge and day 30.
Conclusions: A dedicated two stent strategy utilising the Tryton Side-Branch StentTM to treat bifurcation lesions is feasible showing good acute and 30 day results.
Introduction
Advances in the technology and techniques associated with percutaneous coronary interventions (PCI), specifically balloon expandable stents, have provided the means to treat the wide spectrum of patho-anatomy routinely encountered by the interventional cardiologist. Currently available stents were designed for straight lesions, optimised to provide scaffolding (coverage and radial strength) and ease of deliverability. In straight lesions, these stents have been shown to provide superb acute and long-term results1-3. One lesion subset that continues to challenge the interventionalist is bifurcations lesions. A number of different strategies have been employed with standard stents to address bifurcation lesions each of which have significant limitations4. Large contemporary registries characterising current stent usage in bifurcating lesions have demonstrated decreased procedural success with increased rates in restenosis and thrombosis (acute, subacute and delayed)5,6.
The limitations of currently available stents have led groups to develop stents designed specifically to treat bifurcation lesions. The Tryton Side-Branch StentTM (Tryton Medical, Inc., Newton, MA, USA) is a balloon expandable cobalt chromium stents, designed specifically to treat bifurcation lesions7. We report the acute and 30 day results from the Tryton I first-in-man (FIM) study, a multicentre prospective single arm study evaluating the safety and feasibility of the Tryton Side-Branch StentTM when used in conjunction with standard drug eluting stents to treat de novo bifurcation lesions within the coronary vasculature.
Methods
Tryton Side-Branch StentTM
The Tryton Side-Branch StentTM (Tryton Medical, Inc., Newton, MA, USA) is a slotted tube, balloon expandable cobalt chromium bare metal stent with three zones: a ‘distal side-branch zone’, a ‘central transition zone’ and a ‘proximal main vessel zone’7. The distal side-branch zone has the design characteristics of a standard slotted tube workhorse stent. The central transition zone has a specific geometry, which contains three panels, each of which can be deformed in an independent fashion. The proximal main vessel zone is composed of three fronds that are connected proximally to the transition zone panels and terminate in a circumferential band. The Tryton Side-Branch StentTM evaluated in this study is 18 mm long (distal side-branch zone [6 mm], central transition zone [4 mm], proximal main vessel zone [8 mm]) with a strut thickness of 0.003 inches. The Tryton StentTM was provided on one of two stent delivery systems (straight balloon and stepped balloon). The straight balloon-stent delivery system is of standard tubular construction, and when inflated to 8 atm, has a constant diameter of 2.5 mm. The stepped balloon-stent delivery system has an inflated geometry that corresponds to the three Tryton stent zones (proximal main vessel zone diameter = 3.5 mm, distal side branch zone diameter = 2.5 mm at nominal inflation pressure). Both stent delivery systems have a total of four markers. In addition to standard end markers, delineating the proximal and distal ends of the stent, two additional markers identifying the proximal and distal extent of the transition zone are present.
Tryton Side-Branch StentTM delivery protocol
The Tryton Side-Branch StentTM is used in conjunction with as workhorse (main vessel) stent deployed using standard PCI techniques. The lesion is predilated according to the operator’s discretion leaving a guidewire in both the side-branch and in the main vessel. A Tryton Side-Branch StentTM mounted on a dedicated stent delivery system is then positioned with the central transition zone markers straddling the side branch origin. The Tryton Side-Branch StentTM is deployed after which the stent delivery system is removed. The guidewire initially placed in the side branch is then repositioned in the distal main vessel. A standard main vessel stent is then tracked through the proximal main vessel zone of the Tryton Side-Branch StentTM, into the distal main vessel. The main vessel stent is then deployed, after which the main vessel stent delivery balloon is removed. The side branch is re-accessed after which a simultaneous kissing balloon post-dilatation is performed. This procedure can be performed through a 6 Fr guide.
Study design
The Tryton I (FIM) study protocol was approved by the appropriate medical ethics committees. All patients enrolled provided written informed consent. The Tryton I (FIM) study is a multicentre, prospective, single arm study designed to assess the safety and feasibility of the Tryton Side-Branch StentTM when used in conjunction with a standard main vessel to bifurcated de novo lesions within the native coronary arteries.
Patient population
Patients were offered participation and recruited for the study if they were either clinically stable with angina (stable or unstable), or presented objective evidence of ischaemia with angiography demonstrating a high grade de novo coronary bifurcation lesion involving a main vessel (MV) with a reference vessel diameter of 2.5-5.0 mm and side branch (SB) reference vessel diameter of 2.25-2.75 mm by visual estimate. Patients were excluded if the lesion was within the left main coronary artery, or had been previously treated. Lesions were treated following the protocol outlined above using a Tryton Side-Branch StentTM for the side branch and a drug eluting stent (TAXUS® Paclitaxel-Eluting Coronary Stent, Boston Scientific, Billerica, MA, USA or Cypher Select® Sirolimus-eluting Coronary Stent, Cordis Corporation, Miami Lakes, FL, USA) of the appropriate sizes in the main vessel. Selection of the straight-balloon stent delivery system or the stepped-balloon stent delivery system was left to the operator’s discretion. Intravascular ultrasound (IVUS) was performed at the completion of the index procedure and at 6 months. Clinical follow-up by telephone was performed at 30 days and at 9 months following index procedure. All patients received aspirin, clopidogrel and heparin as per the routine used at each specific site. Patients were continued on dual antiplatelet therapy for a minimum of 6 months.
Study endpoints
The primary study endpoint is defined as procedural success without Major Adverse Cardiac Events (MACE) during the index hospitalisation. Angiographic success was achieved if there was < 30% residual stenosis in main vessel (MV) and side branch (SB) with TIMI 3 flow in both vessels post-procedure. Absence of in-hospital MACE includes: cardiac death; stroke; myocardial infarction; emergent coronary artery bypass graft surgery; and target lesion revascularisation. A myocardial infarction was defined as either the advent of new abnormal Q-waves in accordance with the Minnesota Code for pathologic Q-waves, or if creatinine kinase (CK) was twice the upper normal limit and there was a rise in the level of MB iso-enzyme of CK (CK-MB). Target lesion revascularisation (TLR) was defined as a repeat treatment of a lesion located within the index coronary segment. Target vessel revascularisation (TVR) was defined as a revascularisation of any segment of the index coronary artery. A data safety monitoring board was established to review all MACE events.
Secondary endpoints included clinical, safety and angiographic parameters. MACE and anginal status were assessed at one month together with revascularisation. Safety parameters included major bleeding and major vascular complications. Angiographic success was determined by off-line quantitative coronary angiography (QCA) pre- and post-procedural at the Institut Cardiovasculaire Paris Sud in Massy, France.
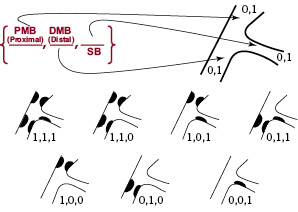
Figure 1. The Medina classification of bifurcation lesions
Results
Baseline data
DEMOGRAPHICS
Although a total of 30 patients were included in the study, full demographical data was available for only 27 at the time of this publication. The average age of the patients was 66 years, with the majority being male (63%) and overweight (BMI=27.6). Nearly one-fifth (19%) of the patients were diabetic and over a third (37%) were currently smokers. Both hypercholesterolaemia and hypertension had a relatively high prevalence (70% each). There where eight patients with previous MI, with one patient having a previous CABG and nine patients (33%) having prior PCI. The prevalence of peripheral vascular disease was relatively low (two patients). The majority of patients had angina (93%), with 41% having stable anginal syndrome (Canadian Cardiac Society Class II), and 37% were categorised as unstable angina. All patients were on aspirin and over 85% on clopidogrel prior to the procedure.
ANGIOGRAPHIC CHARACTERISTICS
Baseline angiographic characteristics are presented in Table 1.
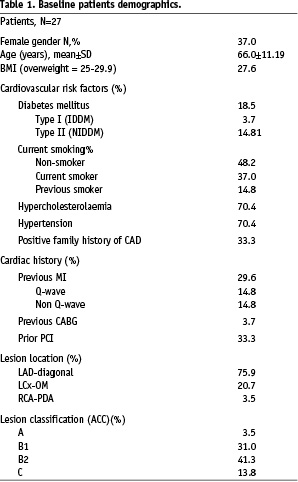
Index lesions in the majority of patients were located within the left anterior descending artery (LAD) involving a major diagonal branch (20 patients, 74%), with six (22%) patients having a diseased left circumflex artery involving a large obtuse marginal branch (Lcx-OM), with only one having disease within the right coronary artery (RCA) involving the posterior descending branch (PDA). The lesions were categorised using the modified ACC classification: 13.8%, class C; 41.3%, class B2; 31.0%, class B1 and 3.5%, class A8. The lesions were classified in accordance with the Medina classification for bifurcations. This classification utilises a three-digit binary key to designate absence (‘0’) or presence (‘1’) of disease in the proximal main vessel, distal main vessel, or side branch. For example, a Medina class 1,0,1 indicates a lesion with angiographic disease present within the proximal main vessel, absent within the distal main vessel and present in the side branch9. Medina classification by visual estimation was available in all 30 patients: 5 (17%) patients had disease in all segments corresponding to a classification of 1,1,1; 10 (33%) patients had disease in either the proximal or distal MB as well as disease in the SB (Medina 1,0,1 or 0,1,1); 14 (47%) patients had MB disease with no disease evident within the SB (Medina 1,1,0, or 1,0,0 or 0,1,0) and one (3%) patient had disease limited to the SB (0,0,1). In total, 15 patients (50%) had disease in both the MB and SB (1,1,1 or 0,1,1 or 1,0,1). Assessment of the bifurcation angulation (between the SB and MB) revealed that in five (17%) patients the angulation was less than 49°, in 21 (70%) patients it was between 45° and 90°, and it was more than 90° in four (13%) patients.
Procedural results
A total of 30 patients were entered into the study. Two patients met criteria for MACE events resulting in a 93.3% success rate. In one patient, the Tryton Side-Branch StentTM failed to track to the lesion site resulting in 96.7% angiographic success rate.
The stepped balloon-stent delivery system was used in 61% of the procedures with the straight balloon stent-delivery system used in the remaining 39%. All procedures were performed via 6 Fr guides. During the initial cases, difficulty in advancing the main vessel stent through the Tryton StentTM was noted. Specifically, it appeared that the leading edge of the main vessel stent was held up as it was exiting the Tryton Side-Branch StentTM. The main vessel stent was removed without difficulty after which the deployed Tryton Side-Branch StentTM was ‘pre-dilated’ with a standard balloon, sized to the main vessel. The main vessel stent was then tracked through the Tryton StentTM without difficulty. It was suggested that ‘pre-dilatation’ of the Tryton StentTM prior to placement of the main vessel stent be performed in subsequent cases. In all subsequent cases the main vessel stent was delivered without difficulty. Side branch re-access followed by simultaneous kissing balloon ‘post-dilatation’ of the MB and SB was achieved in 100% of the cases
in which a Tryton Side-Branch StentTM had been deployed.
MACE EVENTS
Two patients experience MACE events:
The first patient had a highly angulated calcified left anterior descending-diagonal bifurcation (LAD-Diag) lesion. The initial attempt to track the Tryton Side-Branch StentTM to the lesion site was unsuccessful. The Tryton Side-Branch StentTM was then removed without difficulty. Subsequent balloon dilatations were performed. Coronary perforation was noted immediately following a simultaneous kissing balloon inflation requiring emergency pericardiocentesis and placement of a covered stent. The patient was stabilised and transferred to the cardiac care unit. The patient subsequently decompensated and was brought back to the cardiac catheterisation suite where efforts to resuscitate him were unsuccessful.
The second patient who encountered a MACE event had a lesion within the LAD involving a large diagonal branch. During the procedure, the patient was noted to have angina along with EKG changes on the monitor. Serial angiography demonstrated TIMI III flow in the index vessel, however a moderate size ramus intermedius (RI) was noted to be occluded in the mid-portion. Activated clotting time performed at this time was noted to be within normal limits. To re-establish flow in the ramus intermedius (RI), a stent was placed. The index artery was successfully treated following the Tryton protocol. The remainder of the patient’s hospital course was unremarkable, however the CPK was noted to rise 2-3 times baseline qualifying as a MACE event.
Secondary endpoints: 30-day clinical, safety, angiographic and QCA
Follow-up safety data was obtained for all 30 patients at 30 days (Table 2).
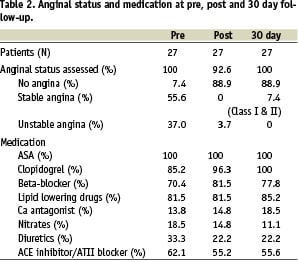
Clinically, 82% (25/27) patients were angina free at follow-up with two patients reporting stable CCS Class I /II symptoms. All patients were on aspirin and clopidogrel. There were no reported cerebrovascular or bleeding events. Between discharge and day 30 there was no additional MACE events identified. Patients did experience non-MACE serious adverse events (SAE’s) including a non-protocol angiogram for evaluation of chest pain which demonstrated persistence of good angiographic results. Two patients underwent further revascularisations of lesions distant from the index lesion. Other noncardiac SAE’s occurring during follow-up included a patient diagnosed with deep femoral vein thrombosis and a patient with a minor vascular access complication (Table 3).
Results of the QCA is presented in Table 4.
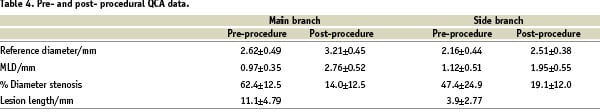
Analysis of the QCA of the MB showed an average lesion length was 11.08±4.79 mm with vessel reference diameter of 2.62±0.49 mm increasing to 3.21±0.45 mm after treatment. The minimum luminal diameter (MLD) increased from 0.97±0.35 mm to 2.76±0.52 mm and was associated with a 48% reduction in the percent of diameter stenosis along the lesion. The average length of the SB segment at baseline, was 3.9±2.77 mm, with a vessel reference diameter of 2.16±0.44 mm. The MLD at the SB increased from 0.97±0.35 mm to 2.76±0.52 mm (Table 4).
Discussion
The treatment of bifurcation lesions continues to be a major problem confronting the interventional cardiologist. In the past decade many different approaches have been proposed to address bifurcation disease. Novel, multi-stent approaches including ‘T’, ‘Y’, culotte, ‘crush’ and ‘double-barrel’ have been described and to varying degrees studied4. As a group, two-stent procedures are often difficult to perform and have been associated with increased thrombosis and restenosis4. These techniques are limited by the design of currently available stents, which were optimised for straight, non-bifurcating lesions. The experience from nearly two decades of stent development has shown that uniform distribution of stent struts with minimal stent overlap are key ingredients to early and late efficacy. None of the current one- or two-stent techniques provide a means to predictably obtain uniform strut coverage at the side branch ostium. The poor long term outcomes with the ‘crush’ technique is likely driven by the excessive (three) layers of metal, with incomplete apposition, especially when there is difficulty recrossing and performing a final kissing balloon inflation10. With ‘T’ stenting, limited results are likely driven by the inability to reliably position the side branch stent with the precision necessary to perfectly cover the side branch ostium, without either leaving a gap (deployment too distal) or having the stent protrude into the main vessel lumen (deployment too proximal). Furthermore, adequate coverage is only possible when the side-branch-to-main vessel angle is nearly 90°11,12. The results of studies evaluating dedicated two-stent strategies have driven many operators to elect a ‘provisional’ single stent strategy as the lesser of two evils. The limitation of current stent design and deployment strategies has been highlighted recently by studies comparing dedicated two-stent strategies to single stent ‘provisional’ strategies13. Nevertheless, provisional stenting is associated with all the uncertainty of ‘POBA’ (plain old balloon angioplasty), including frequent compromise of side branch flow, uncertainty of the durability of the initial results and the frequent use of additional stents.
The Tryton Side-Branch StentTM is one of a number of next generation stents designed specifically for the treatment of bifurcation lesions7. The key design consideration was to provide the operator with stent/stent delivery system which has the performance characteristics of a state-of-the-art workhorse stent, e.g., trackability and guide compatibility, while providing uniform strut coverage to the side branch ostium with minimal stent-strut overlap. The strategy of stenting the side branch first provides a means to definitively treat the side branch without compromising with the operator’s treatment strategy, i.e., the stent selection for the main vessel. The results from this study demonstrates the feasibility of this approach.
Safety
The primary purpose of the Tryton I (FIM) study was to demonstrate safety and clinical feasibility. When evaluating the data, it is important to address the two MACE events. The first patient experiencing a MACE event had a coronary perforation, with subsequent tamponade and vessel re-closure leading to the patient’s death. This patient was included in the intention-to-treat cohort because of the initial failure of the Tryton Side-Branch StentTM to track to the lesion site. It is important to note that the Tryton stent-balloon delivery system was successfully removed. Furthermore, the complication occurred immediately following the simultaneous kissing balloon inflations. The second patient who experienced MACE was due to an arterial segment distant from the index artery. Both of these MACE events were reviewed by the DSMB and determined not to be directly attributable to failure of the Tryton Side-Branch StentTM stent delivery system, or the strategy employed.
Feasibility
The data we report here demonstrates that excellent angiographic results can be achieved using the Tryton Side-Branch StentTM via a 6 Fr guide. The high rate of procedural and angiographic success are encouraging. Furthermore, the ability to re-cross and complete each case with a simultaneous kissing balloon inflation, highlights the ease which this procedure can be performed. The importance of the final kissing balloon inflations has been validated by the multiple series evaluating the ‘crush’ technique14. It is anticipated that the Tryton Side-branch StentTM could achieve superior results due to the single layer of struts coverage at the side branch ostium. The 6 month angiographic and IVUS follow-up will provide insights into the durability of the results observed, and the necessity of adding DES capabilities to future generations of the Tryton StentTM.
Limitations
The Tryton I (FIM) study is a small study designed to examine procedural safety and clinical feasibility. It does not have the statistical power to address issues such as subacute thrombosis.
Conclusion
The acute and 30 day results from the Tryton I (FIM) study demonstrate that safety and feasibility of a dedicated side-branch strategy with the Tryton Side-Branch StentTM when it is used in conjunction with a standard workhorse drug eluting stent to treat complex bifurcation lesions within the coronary vasculature. Larger studies with long-term data will be required to fully understand the utility of this approach.
Acknowledgements
The Tryton I (FIM) Study was financed in part by Tryton Medical, Inc.
The authors wish to acknowledge research staff at each of the study sites who’s contributions to this study were essential:
Helios Heart Center, Siegburg, Germany: Eberhard Grube, M.D. (Principal Investigator), Ralf Müller, MD, Jucelimo Schmitt, Kerstin Kupfer, RN.
Thoraxcenter, Erasmus University, Rotterdam, The Netherlands: Patrick W. Serruys, MD, PhD (Principal Investigator), Robert Jan van Geuns, MD, Wim van der Giessen, MD, PhD, George Sanos, MD, Steve Ramcharitar, DPhil, BMBCh, MRCP; Maarten Vermeulen, RN; Arno Ruiter, RN.
Institut Cardiovasculaire Paris Sud, Massy, France: Marie-Claude Morice, MD (Principal Investigator), Yves Louvard MD, Thierry Lefeyre, MD, Béatrice Beaurain RN.
Data Safety Monitoring Board
Antonio L. Bartorelli, MD. Institute of Cardiology of the University of Milan, Centro Cardiologico “Monzino”, IRCCS, Milan, Italy.
Dean J. Kereiakes, MD. The Heart Center of Greater Cincinnati and The Lindner Center at The Christ Hospital, Cincinnati, OH, USA.

