Abstract
Aims: High-risk plaques are prone to develop at the site of coronary vessel bifurcations. The distribution of necrotic core at bifurcation lesions (BL) is known, however, little has been described on the necrotic core distribution in non-BLs. Therefore we compared the distribution of necrotic core between BL and non-BLs in coronary arteries using IVUS-VH imaging.
Methods and results: A total of 129 patients (112 non-BL and 108 BL) were included. The lesions were divided into upstream and downstream segments according the location of the minimum lumen area (MLA) within the plaque. In BLs, compositional analysis showed no differences between the three segments. The necrotic core in contact with the lumen that was located in the downstream segment was significantly larger. While in non-BLs, this was not significantly different between segments. Plaque burden in BLs was 56.60±5.79% vs. 55.50±4.54% in non-BLs, p=0.04. Mean necrotic core area was larger in BLs 0.84±0.55mm2 vs. 0.70±0.49mm2, p=0.048. Mean percentage necrotic core was 15.48±8.02% vs. 14.51±7.64%, p=0.37. There was a trend towards a greater content of necrotic core in contact with the lumen in BLs. The percentage of frames with a major confluent pool of necrotic core in contact with the lumen >10% in BLs was 11.78±17.18 vs. 8.95±17.86 in non-BLs, p=0.065. There was a statistically significant difference in the frequency of IVUS derived thin capped fibroatheromas between bifurcation lesions 20 vs. 13 in non-bifurcation lesions, p=0.03.
Conclusions: Bifurcation lesions appear to have a larger plaque burden with a different plaque composition compared to non-bifurcation lesions. This may partly explain the adverse outcomes seen following treatment of bifurcation lesions in contemporary practice.
Introduction
Atherosclerotic plaque is the pathognomonic characteristic of the early stages of atherothrombosis, the final consequence of vascular inflammation, cholesterol deposition and thrombus formation. The identification of sub-clinical high-risk plaques (i.e., necrotic core rich plaques) using intra-vascular ultrasound-virtual histology (IVUS-VH) is potentially important because these plaques may have a greater likelihood of rupture and subsequent thrombosis.1 Studies indicates that these high-risk plaques are prone to develop at the site of coronary vessel bifurcations due to the specific shear stress conditions present in these regions. For example an in vivo study of 103 bifurcations, using IVUS-VH and optical coherence tomography, found that the per cent of necrotic core at the proximal rim, bifurcation and distal rim was 16.8%, 15.2% and 13.5%, respectively (p<0.001). Contrary to this, little is presently known about the necrotic core distribution in non-bifurcation lesions.2
Therefore we compared the distribution of necrotic core between bifurcation and non-bifurcations in coronary arteries using IVUS-VH imaging.
Material and methods
Patient selection
The Volcano Registry is a global, prospective, multi-centre (42 centres), non-randomised study that started in March 2004, and enrolled 990 patients with coronary artery disease who had IVUS-VH performed during coronary catheterisation. For this substudy, all patients were screened for eligibility: presence of a side branch (>1.5 mm in diameter) within the imaged segment. Eventually 129 patients were selected who were older than 18-years of age.
The local ethical committee of each participating centre approved the protocol and informed written consent was obtained from all patients.
IVUS-VH acquisition and analysis
Imaging acquisition was performed during invasive coronary angiography either during diagnostic procedures, or in the non-culprit vessel during percutaneous coronary intervention (PCI). Briefly, IVUS-VH uses spectral analysis of IVUS radiofrequency data to build tissue maps that are correlated with a specific spectrum of the radiofrequency signal and assigned colour codes (fibrous [labelled green], fibrofatty [labelled greenish-yellow], necrotic core [labelled red] and calcium [labelled white]).5
In the Volcano Registry IVUS-VH data was acquired using the Eagle-Eye™ 20 MHz catheter, (Volcano Therapeutics, Rancho Cordova, CA, USA) and a continuous pullback by a dedicated IVUS-VH console (Volcano Therapeutics, Rancho Cordova, CA, USA). The IVUS-VH data were then stored on a DVD and sent to an independent Corelab (Cardialysis, Rotterdam, The Netherlands) for offline analysis. Data acquisition was ECG-gated and recorded during the automated withdrawal of the catheter using a mechanical pullback device (TrakBack II/R-100 Volcano Therapeutics, Rancho Cordova, CA, USA) at a pullback speed of 0.5 mm/s. Geometrical and compositional data were obtained for every slice. The contours of the external elastic membrane (EEM) and the lumen-intima interface enclosed an area that was defined as the coronary plaque plus media area. Plaque burden was defined as ([EEMarea-Lumenarea]/EEMarea) x 100. Direct measurements (lumen and vessel cross-sectional areas – [CSA]) were also determined. Quantification of the necrotic core in contact with the lumen was performed using MATLAB® (MathWorks, Natick, MA, USA).
Bifurcation lesions analysis
All consecutive frames with a plaque burden ≥40% around a side branch ≥1.5mm in diameter were considered part of the lesion. This lesion was divided into upstream and downstream segments according the location of the minimum lumen area (MLA) within the plaque. Imaging was only performed in the main branch (Figure 1).
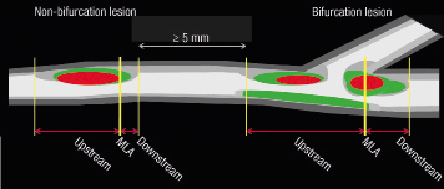
Figure 1. The bifurcation and non-bifurcation coronary lesions assessed in this study. The lesions were divided into three sub-segments: Upstream, minimum luminal area (MLA) and downstream sub-segments. Please note that the lesions should be more than 5 mm apart form each other.
Non-bifurcation lesion analysis
These lesions were defined as follows: all consecutive frames with plaque burden ≥40% were considered as part of the non-bifurcation lesion. Lesions in the same coronary segment were required to be at least 5 mm apart to be considered separately. Within these lesions, the frame with the MLA was selected as the divider of the plaque, that is the plaque was divided into the upstream (proximal to the MLA) frame and the downstream segments (distal to the MLA) frame (Figure 1).
In general, only lesions that have upstream and downstream frames were included for the comparison between these two segments.
Definitions
Necrotic core tissue in contact with the lumen (NCCL), defined as the presence of necrotic core tissue in direct contact with the luminal space and with no detectable overlying fibrous tissue; it was reported (i) as percent of the total plaque composition and (ii) as a percent of the total necrotic core. In addition, the major confluent pool of NCCL (MCNC) was selectively quantified as a percentage of both the total plaque composition and the total necrotic core (Figure 2).3,4
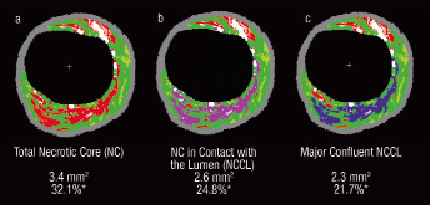
Figure 2. This figure illustrates the detailed quantification of the necrotic core that is in contact with the lumen. a) Total necrotic core is in red; b) Necrotic core in contact with the lumen is in violet; c) Major confluent necrotic core in contact with the lumen is in blue. *%necrotic core of plaque area
IVUS-derived TCFA (IDTCFA), defined as a lesion fulfilling the following criteria in at least three consecutive CSAs : (i) plaque burden ≥ 40%; (ii) confluent necrotic core ≥ 10% in direct contact with the lumen in the investigated CSA (Figure 2); all consecutive frames having the same morphologic characteristics were considered as part of the same IDTCFA lesion.
The percentage of frames per lesion having more than 10% MCNC in contact with the lumen was calculated as follows: number of frames in the lesion with >10% MCNC in contact with the lumen divided by their counterparts X 100.
Statistical analysis
Discrete variables are presented as counts and percentages. Continuous variables are presented as means ±1standard deviation. The distribution of the different variables were tested with Kolmogorov-Smirnov and in case of non-normal distribution non-parametric statistics were used to calculate p values for the comparison between two groups.
The bifurcation and non-bifurcation (lesions) were the units of analysis without corrections for correlated observations in the same subjects.
A two-sided P value <0.05 was required for statistical significance. All analyses were performed using SPSS version 15.0 software (SPSS Inc., Chicago, IL, USA).
Results
A total of 129 patients (112 non-bifurcation lesions and 108 bifurcation lesions) were included. The baseline characteristics of the patient population are shown in Table 1.
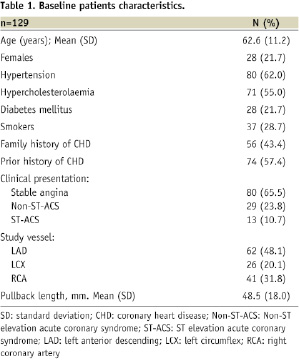
The mean age was 62.6±11.2 years, most being male patients (78.3 %). The vessel of interest was the left anterior descending (LAD) in 48.1%, the left circumflex (LCX) in 20.1% and the right coronary artery (RCA) in 31.8% patients. Patients presented with stable angina 65.5% and 23.8% had non-ST segment elevation acute coronary syndrome.
The locations of the bifurcation lesions were as follows: LAD/Diagonal=39, LAD/septal=12, LCX/1st obtuse marginal (OM)=17, LCX/2nd OM=3, RCA/ventricular branch=14, RCA/posterolateral=5.
IVUS-VH findings in bifurcation lesions
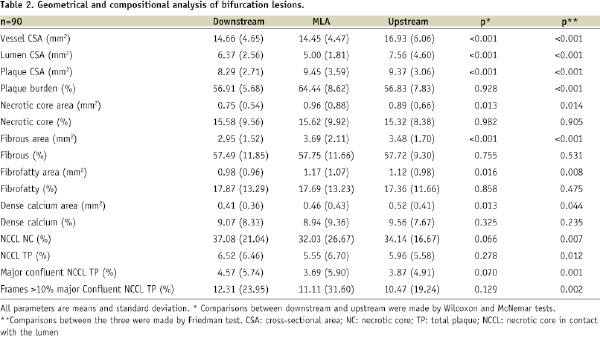
In Table 2, the geometrical and composition analyses are shown of the 90 bifurcation lesions which had upstream and downstream frames.
The overall mean length of the bifurcation lesions was 14.62±14.24 mm, whilst the upstream and downstream segment lengths were 9.09±11.90 mm and 5.03±3.00 mm, respectively.
As expected, the MLA frame had the largest plaque burden as compared to the other two segments (p<0.001). The vessel CSA at the MLA frame was smaller than the other segments (p<0.001), which was largely due to the location of the MLA frames with respect to the side branch (25.3% of the MLA frames were located proximal to the side branch of the bifurcation, 21.8% at the side-branch site and 52.9% distally).
Overall, the relative compositional analysis showed no differences between the three segments. Specifically, only the necrotic core in contact with the lumen that was located in the downstream segment was significant.
A total 20 ID-TCFAs were found in these 90 bifurcation lesions. Five of them involved upstream as well as downstream segments, whilst among the remaining, eight were located upstream and seven downstream (p=0.9).
IVUS-VH findings in non-bifurcation lesions
Out of 112 non-bifurcation lesions, 95 had upstream and downstream frames. The geometrical and composition analyses are shown in Table 3.
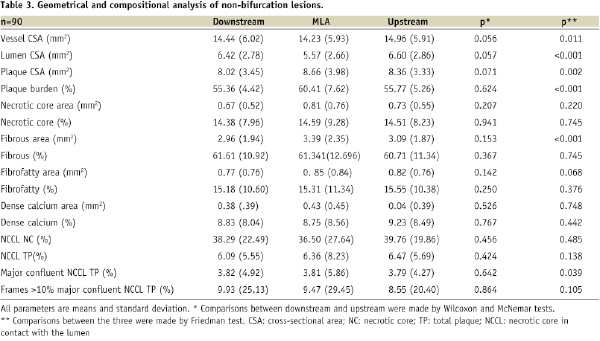
The overall mean length of the non-bifurcation lesions was 9.84±9.65mm, whilst the upstream and downstream segments measured 5.47±7.66mm and 3.88±5.97mm respectively.
Similarly to bifurcation lesions, the MLA frame had the largest plaque burden as compared to the other two segments (p<0.001). Interestingly, the vessel CSA at the MLA frame was smaller than the other segments which may imply constrictive remodelling in this part of the plaque (p=0.011). Again the compositional analysis showed no differences between the three segments. Of note, although not significant, the amount of necrotic was larger in the upstream segment and the MLA, as compared to the downstream segment.
The necrotic core in contact with the lumen was not significantly different between the three segments. A total 13 ID-TCFAs were found in these 95 non-bifurcation lesions. Nine of them were located in the upstream part of the lesion (p=0.267).
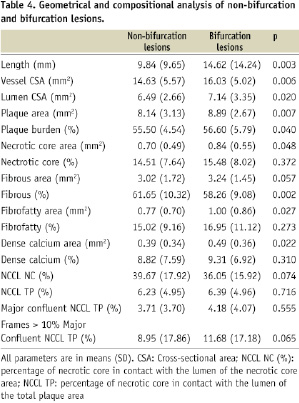
IVUS-VH findings in bifurcation as compared to non-bifurcation lesions
Vessel CSA was larger in bifurcation lesions as compared to non-bifurcations lesions (16.03±5.02 mm2 vs. 14.63±5.57 mm2, p=0.006). Lumen and plaque cross-sectional areas were also larger in bifurcation lesions as compared to non-bifurcations lesions (7.14±3.35 mm2 vs. 6.49± 2.66 mm2, p=0.02 and 8.89±2.67 mm2 vs. 8.14±3.13 mm2, p=0.007, respectively). Plaque burden in bifurcation lesions was 56.60±5.79% vs. 55.50±4.54%, p=0.04. Overall mean necrotic core area was larger in bifurcation lesions 0.84±0.55 mm2 vs. 0.70±0.49 mm2, p=0.048. Adjusted for plaque size, mean percentage necrotic core was 15.48±8.02% vs. 14.51±7.64%, p=0.37. There was a trend towards a greater content of necrotic core in contact with the lumen in bifurcation lesions. The mean proportion of frames with MCNC >10% in bifurcation lesions was 11.68±17.18 vs. 8.95±17.86 in non-bifurcation lesions, p=0.065. This resulted in a statistically significant difference in the frequency of ID-TCFAs between bifurcation lesions 20 vs. 13, p=0.03 (Table 4).
Discussion
The main findings of this study were the following: (i) In bifurcation and non-bifurcation lesions there are no significant difference in compositional analyses between upstream and downstream segments; (ii) Bifurcation lesions have a significantly larger plaque burden and a larger area of necrotic core in contact with the lumen. (iii) This resulted in a greater number of IDTCFAs in bifurcation versus non-bifurcation lesions.
In general the content of necrotic core and plaque composition was comparable between upstream and downstream segments in bifurcation and non-bifurcation lesions. These results are in line with our previous findings which demonstrated that plaque rupture was present in 39.3% of upstream plaque, 21.4% of plaque at the MLA and 32.1% of downstream plaque.5
Important geometrical differences have been found between the non-obstructive bifurcation and non-bifurcation lesions evaluated in this study. Notably lesion length varied significantly between lesions (14.6 mm bifurcation vs. 9.8 mm non-bifurcation), and consequently the upstream and downstream segment lengths were different – the upstream segment was almost twice as long in bifurcation lesions (9.09 vs. 5.47 mm), with only a 1.1% absolute difference in %plaque burden (1.9% relative difference); this suggests a very different geometry in bifurcation and non-bifurcation lesions. With such a marked difference in lesion geometry, and by association, shear stress, one would expect a greater difference in plaque composition between lesion types. Some reports have suggested that shear stress may play a role in both the distribution of the plaque, and the distribution of components within the plaque.6 The geometry of an encroaching atherosclerotic plaque has an impact on the pattern of the blood flow. Indeed, proximal to the MLA i.e., at upstream endothelium sites the plaque is under high-shear stress, whereas at downstream sites low-shear stress prevails. Furthermore, atherosclerotic plaque composition varies greatly,7 with a higher concentration of macrophages upstream, whilst smooth muscle cells (SMCs) predominate in the downstream part of the atherosclerotic plaque.7,8 At present there is no overall consensus on the role of shear stress on either plaque composition or, as a trigger factor for plaque rupture. Further investigation is warranted.
To the best of our knowledge, this is the first in vivo study comparing geometrical and compositional analyses in bifurcation and non-bifurcation lesions. Bifurcation lesions account for approximately a quarter of lesions undergoing PCI, and have consistently been associated with poorer outcomes when compared to non-bifurcation lesions, although the reasons behind this are not entirely apparent.9,10 Previously the use of two stents was blamed, however data continues to indicate that poorer outcomes persist even in patients treated with one-stent strategies. Tsuchida et al reported a MACCE rate of 14.1% in patients with bifurcation lesions treated with one-stent compared to 11.0% in patients without bifurcation lesions.11 Other possible causes for these poorer outcomes include the plaque burden at bifurcations sites, which may consequently influence restenosis as has been demonstrated in patients with diabetes. Diabetes not only results in a greater plaque burden,12 but has also consistently been identified as an independent predictor of restenosis after PCI with either bare metal or drug eluting stents.13,14 Another possibility is that the restenosis is related to the composition of the underlying plaque. Previously in carotid arteries Hellings et al demonstrated a positive correlation between plaque composition and rates of restenosis after stenting.15
Two studies have evaluated the usefulness of IVUS-VH plaque composition to predict the risk of embolisation during stenting16,17 In one of these studies Kawamoto et al used a Doppler guidewire in 44 patients undergoing elective PCI to record the high-intensity transient signals (HITS) produced by the small embolic particles that were liberated during stenting. Patients were then divided into the tertiles according to the HITS counts. Dense calcium and necrotic core area were significantly larger in the highest tertile. Moreover, in the multivariate logistic regression analysis, only necrotic core area was an independent predictor of a high HITS counts (odds ratio 4.41, p=0.045). The greater plaque burden and percentage of necrotic core seen in bifurcation lesions may therefore account for the increased risk of periprocedural MI during treatment of bifurcation lesions.10 Moreover, the use of plaque stabilising medication such as statins may reduce this risk, as suggested by the recent ARMYDA-RECAPTURE study which demonstrated, albeit not specifically in bifurcation lesions, a reduction in periprocedural myocardial infarction (p=0.056) following the reloading of patients with 80 mg of atorvastatin.18
Limitations of the study
There are a number of limitations associated with the present study. Firstly, no imaging was performed in the side branch of the bifurcation lesion. Moreover, we acknowledge that there was no independence between the observations, such that bifurcation and non-bifurcation lesions may have come from the same patient. These observations are only related to non-obstructive lesions; the size and composition may be different in obstructive plaques. The number of high-risk plaques (i.e., TCFAs) is low and although their geographic spread is reported here, no definitive conclusions can be drawn on this, primarily because this study does not represent the whole spectrum of the disease.
Conclusions
Bifurcation lesions appear to have a large plaque burden and different plaque composition compared to non-bifurcation lesions, and this may partly explain the adverse outcomes seen following treatment of bifurcation lesions in contemporary practice.
Acknowledgements
Funding/Support: This study was funded by Volcano Corporation.
Role of the Sponsor: The sponsor participated in discussions regarding study design and protocol development and provided logistical support during the trial.
Database was performed by a contract research organisation, Pacific Data Design, California, US, under contract to the sponsor.
VOLCANO REGISTRY Investigators and participating centres:
United States: Jason Rogers, MD, UC Davis Medical Center, Sacramento, CA; Steve Marso, MD, Mid-America, Kansas City, MO; Jim Margolis, MD, Miami Heart, Miami, FL; Amir Lerman, MD, Mayo Clinic, Minneapolis, MN; Juan Pittaluga, MD,Winchester Medical, Winchester, VA; George Revtyak, MD, St. Francis Hospital, Beech Grove, IN; Ed O’Leary, MD, Nebraska Heart, Omaha, NE; Murat Tuzcu, MD, Cleveland Clinic Foundation, Cleveland, OH; Nabil Dib, MD, Arizona Heart, Phoenix, AZ; Rajesh Dave, MD, Pinnacle Health
Harrisburg, PA; Morton Kern, MD, Saint Louis University, Saint Louis, MO; John Hodgson, MD, Saint Joseph’s Hospital, Phoenix, AZ; Samer Kazziha, MD, Mount Clemens General, Mt. Clemens, MI; Greg Braden, MD, Forsyth Hospital, Forsyth, NC; Louis I. Heller, MD, Saint Joseph’s Hospital, Atlanta, GA; Marty Leon, MD, Columbia University Medical Center, New York, NY; Joseph Puma, MD, New York Methodist, Brooklyn, NY.
Europe: V. Klauss, MD, Innenstadt, Munich, Germany; M. Pieper, MD, Herz-und Neurozentrum, Kreuzlingen, Switzerland; D. Glogar, MD, AKH, Vienna, Austria; P. Oemrawsingh, MD, LUMC, Leiden, The Netherlands; O. Bleie, MD, Haukeland Hospital, Bergen, Norway; D. Dudek, MD, Jagiellonian University, Krakow, Poland; Schiele, MD, CHU, Besancon, France; M. Sabate, MD, Hopital Clinico San Carlos, Madrid, Spain; Prof. P. Serruys, MD, Thoraxcenter, Rotterdam, The Netherlands; R. Erbel, MD, Westdeutsches Herzzentrum, Essen, Germany; W. Wijns, MD, OLVZ, Aalst, Belgium; Colman, MD, Valdecilla Hospital, Santander, Spain; F. Prati, MD, San Giovannni, Roma, Italy; P. Sganzerla, MD, Cliniche Gavazzeni, Bergamo, Italy; P. Presbitero, MD, Clinica Humanitas, Rozzano, Italy; Miller, MD, Ichilov, Tel Aviv, Israel; R. Kornowski, MD, Rabin Medical Center; Petah Tiqwa, Israel; A. Iniguez, MD, Meixoeiro, Vigo, Spain; E. Eeckhout, MD, CHUV, Lausanne, Switzerland; Harnek, MD, University Hospital, Lund, Sweden; Quininha, MD, Hospital de Santa Marta, Lisbon, Portugal.
Japan: Osamu Katoh, MD, Kenya Nasu, MD, Takahiko Suzuki, Toyohashi Heart Center, Toyohashi; Satoru Sumitsuji, MD, Rinku General Medical Center, Izumisano; Satoshi Saito, MD, Junko Honye, MD, Nihon University, Tokyo.

