Abstract
Aims: In addition to an adjunctive imaging platform during coronary angiography, intravascular ultrasound (IVUS) with Virtual Histology™ (VH) is increasingly being used to quantify coronary atherosclerosis. The relationship between VH-IVUS measures of coronary atherosclerosis and traditional cardiovascular risk factors has not been completely described. The objective of this study was to determine if an association exists between VH-IVUS measures of coronary atherosclerosis and the Framingham risk score in a prospective, multinational registry.
Methods and results: Patients enrolled from 2004-2006 at 37 multinational centres in the prospective VH-IVUS Global Registry were analysed. All subjects underwent diagnostic coronary angiography followed by IVUS. A Framingham risk score (FRS) was calculated for each subject, then stratified into three exclusive estimates (<10%, 10-19%, or ≥20%) for future coronary heart disease (CHD) event risk over 10 years. Among 531 patients, plaque volume of the most diseased 10 mm segment increased with increasing FRS (P=0.006, adjusted for multiple comparisons). Patients with higher FRS estimates of CHD risk had a higher proportion of plaque classified as thin cap fibroatheroma compared with patients in the middle and lower risk score categories (21.4% vs 15.2% and 11.3%, respectively, P=0.008, adjusted for multiple comparisons).
Conclusions: Using data from a large, multinational VH-IVUS registry we describe an association between the Framingham risk score and VH-IVUS measures of atherosclerosis within the most diseased 10 mm segment, namely plaque volume and the proportion of thin cap fibroatheroma.
Introduction
Coronary atherosclerotic complications are the leading causes of worldwide mortality, responsible for >7 million global deaths in 20021. In the United States, 12% of the population has diagnosed coronary heart disease (CHD), resulting in ~4 million annual inpatient hospital admissions2. While models to predict future cardiovascular events use traditional risk factors to estimate the likelihood of complications of coronary atherosclerosis3,4, less is known about the relationship between future risk of vascular events and intravascular ultrasound (IVUS) measures of coronary atherosclerosis.
IVUS is commonly used to optimise device sizing and is increasingly being used in clinical trials to quantify the severity of coronary atherosclerosis in clinical practice and randomised trials5-8. IVUS provides transmural imaging of the coronary artery wall with reproducible measures of coronary atherosclerosis9. Innovation in cardiovascular imaging over the past decade has resulted in the ability to characterise tissue of atherosclerotic lesions. Virtual Histology (VH)™-assisted IVUS is a validated method10-14 that utilises the mathematical technique of autoregressive modelling to classify IVUS-radiofrequency data into one of four colour-coded plaque components: 1) fibrous, 2) fibrofatty, 3) dense calcium, and 4) necrotic core11,13,14. This platform can be extended by defining a specific plaque phenotype using a VH-IVUS image based on a simplified, plaque classification scheme developed by Virmani et al15. Using this nomenclature, non-atherosclerotic intimal deposits are termed “intimal xanthoma” or “adaptive intimal thickening”. Progressive atherosclerotic plaque is classified as pathological intimal thickening, thin cap fibroatheroma (TCFA), fibroatheroma, and fibrocalcific.
The objective of this study was to determine whether VH-IVUS measures of plaque composition are associated with the Framingham risk score (FRS) in the prospective, multinational Global VH-IVUS registry.
Methods
Patient population
Subjects were enrolled during 2004-2006 in the VH-IVUS Global Registry, a prospective registry at 37 multinational centres. Patients underwent coronary angiography followed by IVUS at the discretion of the operator for one or more indications: 1) assessment of an indeterminate lesion, 2) investigation of a culprit lesion for optimal device sizing, 3) post-stent assessment, or 4) clarification of extent of disease. There were 531 patients with analysable VH-IVUS images of native coronary vessels, pullback length >10 mm, and complete clinical and laboratory values to estimate the FRS. Diabetes was determined by reported history or by the presence of active treatment (pharmacologic or diet). Acute coronary syndromes were classified as unstable angina, non-ST-elevation MI, or ST-elevation MI according to American College of Cardiology / American Heart Association guidelines16. Each enrolling centre’s institutional review board approved this study.
IVUS analysis
Qualitative analysis was performed according to the ACC expert consensus on IVUS17. Plaque volume was derived using the Trapezoidal18,19 rule within the defined segment as follows: (external elastic membrane volume - lumen volume).
Vessels were imaged with 20 MHz IVUS catheters (Eagle Eye® Gold, Volcano Corp., Rancho Cordova, CA, USA) during continuous motorised pullback at 0.5 mm/s (R-100TM and TrakBack® II, Volcano Corp.) Software (pcVH V2.2, Volcano) was used to calculate geometric and composition data for each VH-IVUS frame. VH-IVUS is a validated10-14,20,21 method that uses the mathematical technique of autoregressive modelling to classify the IVUS radiofrequency data into one of four colour-coded plaque components for human coronary atherosclerosis: 1) fibrous (green), 2) fibrofatty (light-green), 3) dense calcium (white), and 4) necrotic core (red).
VH-IVUS borders were drawn by technicians at the Cleveland Clinic Department of Biomedical Engineering (Cleveland, OH, USA) or the Cardiovascular Research Foundation (New York, NY, USA) and verified at Cardialysis-Erasmus Research Lab (Rotterdam, The Netherlands). Analyses of the medial-adventitial and luminal borders were performed in each analysable cross-sectional frame for the entire pullback length.
Patients undergoing single-vessel IVUS during diagnostic catheterisation or before percutaneous coronary intervention were included in this analysis. The VH-IVUS region of interest was the most diseased 10 mm segment, identified by summarising plaque volume in contiguous cross sections over an axial distance of 10 mm. The segment with the greatest plaque volume constituted the most diseased 10 mm.
Phenotypic classification
VH-IVUS frames were assigned a phenotype using an automated pixel detection algorithm (Volcano Corp.) based on a modified histopathological classification scheme developed by Virmani et al15 (Figure 1) and expert consensus guidelines for analysing and interpreting VH-IVUS images22. Inter-observer variability in phenotypic classification is removed by the algorithm. Confluence for each compositional element was set at a minimal area for this study. A confluent area was defined as a circular area represented by continuous composition of the same tissue type for a minimum diameter of approximately 12 pixels or 0.08 mm2 on a 400x400 VH-IVUS image. Intimal deposits are described by Virmani et al as either intimal thickening or intimal xanthoma. We referred to this collective entity as adaptive intimal thickening, which was classified as any plaque <600 µm on any VH-IVUS frame. Atherosclerotic plaque consisted of plaque ≥600 µm thick and was assigned to one of four other phenotypes as follows: 1) pathological intimal thickening: predominantly fibrous tissue with or without >15% fibrofatty tissue and without either confluent necrotic core or confluent dense calcium; 2) fibrocalcific: confluent dense calcium without confluent necrotic core; 3) fibroatheroma: confluent necrotic core not at the lumen or, if at the lumen surface, then not exceeding 14 pixels along the circumference of the lumen on three consecutive frames; 4) TCFA: plaque burden >50% and confluent necrotic core extending >14 pixels along the circumference of the lumen on three consecutive frames. These definitions are specific to a VH-IVUS image dimension of 400x400, transcribed by the pcVH software V 2.2.
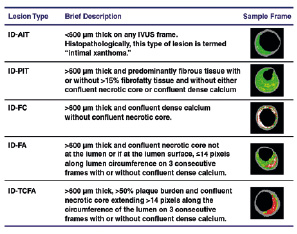
Figure 1. Virtual Histology-IVUS defined classification system for plaque phenotypes. IVUS: intravascular ultrasound; AIT: adaptive intimal thickening, PIT: pathological intimal thickening; FA: fibroatheroma; TCFA: thin cap fibroatheroma; FC: fibrocalcific.
Framingham risk classification
FRS was calculated for each patient using ß-coefficients to compute the linear function as previously described by Wilson and colleagues4. Briefly, the FRS is a validated, gender-specific model that uses age, diabetes mellitus, total or low density lipoprotein cholesterol, high density lipoprotein cholesterol, systolic blood pressure, diastolic blood pressure, and smoking to predict 10-year risk of coronary events including fatal or nonfatal MI or sudden death. Scores were stratified by <10%, 10-19%, or ≥20% 10-year risk for a CHD event.
Statistical analysis
Demographic data were analysed using student t-tests for continuous and X2 tests for categorical data. Individual CHD estimates were calculated using Framingham linear function equations, with subjects stratified by <10%, 10-19%, or ≥20% risk for CHD events over 10-years. Comparisons between Framingham strata were performed using Kruskal-Wallis tests adjusting the P-values for multiple comparisons using a Bonferroni-Holm test. Plaque volume and the TCFA phenotype held significance after the multiple comparison adjustment. To further evaluate plaque volume and TCFA, individual multivariable generalised linear models were developed using a Gamma distribution and Log link function. Estimates and 95% confidence intervals from the models were graphically represented. Statistical significance was defined as P≤0.05. Analyses were conducted using SAS Version 9.1 (SAS Institute, Cary, NC, USA).
RESULTS
Baseline demographics of study patients are shown in Table 1. Left anterior descending was the target IVUS vessel in 261 (49.2%) patients, right coronary artery in 161 (30.3%), left circumflex in 92 (17.3%), and left main in 17 (3.2%). IVUS was performed during diagnostic angiography in 50.6% and prior to PCI in 49.4% of patients.
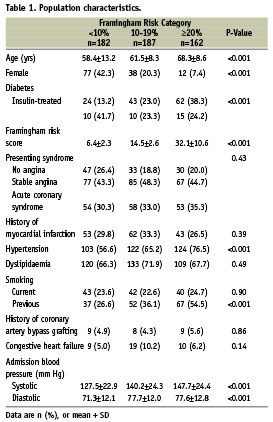
Greyscale IVUS measures of coronary atherosclerosis in the most diseased 10 mm segment increased with increasing FRS (Figure 2A). After adjustment for multiple comparisons, the difference in median [IQR] plaque volume was statistically significant between the <10%, 10-19%, and ≥20% FRS groups (79.9 [65.3-105.4] mm3, 92.7 [73.7-117.4] mm3, and 98.8 [78.2-125.9] mm3, respectively, P< 0.001).
The percentage distribution of plaque phenotypes for the most diseased 10 mm segment is shown in Table 2. Compared with the <10% FRS category, TCFA was nearly two-fold greater in the ≥20% category. The proportion of the remaining plaque phenotypes was similar between FRS categories.
Figure 2 demonstrates the relationship between plaque volume and TCFA by FRS expressed as categorical (A & C) and continuous (B & D) variables. In both cases, both plaque volume and TCFA increased with increasing FRS.
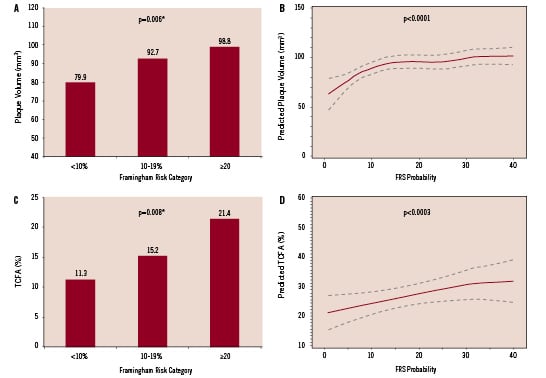
Figure 2. Associations between plaque volume, thin cap fibroatheroma plaque and Framingham risk category. Data in A & C are expressed as categorical variables; B & D display estimated regression line (solid) and 95% confidence interval (dashed). *P-value adjusted for multiple comparisons.
Discussion
Whether a relationship exists between the quantity of coronary plaque and risk of atherosclerotic complications is not known23. There are limited studies linking IVUS measures to future cardiovascular risk24 and no studies to date describing the association between plaque phenotype and future cardiovascular risk. However, there are many IVUS studies evaluating the efficacy of various pharmacologic agents on delaying plaque progression or initiating the process of plaque regression5-8,25-29. There are two novel findings from our work: 1) patients at greater risk for a future cardiovascular event as measured by the FRS have greater plaque volume in the most diseased 10 mm segment; 2) the proportion of TCFA is greater in the most diseased 10 mm segment in patients with higher Framingham risk scores.
The primary outcome variable for this analysis was the Framingham risk score, which was not designed to estimate future cardiovascular risk in a VH-IVUS population but rather to estimate future cardiovascular risk in a primary prevention cohort. However, we believe its use is acceptable for the following reasons: 1) there is no clinical model currently available to estimate future risk of secondary events; 2) it is commonly believed that traditional cardiovascular risk factors associated with the index event will also be associated with risk for secondary events30-34; 3) the FRS was developed and validated in a population of patients without clinically overt heart disease. In fact, 60% of the current study population did not have a history of complications of coronary atherosclerosis at the time of angiography and IVUS, thus these patients may have been eligible in the original FRS validation cohort.
Use of VH-IVUS to define plaque phenotypes may enhance the ability to estimate future cardiovascular risk. Phenotyping allows one to describe a VH-IVUS frame on the basis of plaque composition, confluence of the compositional elements, and the relationship of confluent compositional elements to the lumen and vessel wall. Phenotyping may provide needed clarity in estimating future risk of cardiovascular events. Nevertheless, our findings, specifically with respect to TCFA, should be considered preliminary. Although histopathologically-defined TCFA is associated with sudden cardiac death15 and thus is a logical candidate imaging biomarker to estimate the risk for future death and MI, there are multiple caveats to our use of TCFA. First, VH-IVUS and histopathologically-defined TCFA are not equivalent. Although VH-IVUS is a validated platform to quantify plaque composition, its resolution differs from histopathology. For example, cap thickness of histopathologically-derived TCFA is <65 µm which is beyond the resolution of current IVUS platforms. Second, histopathologic sections of an artery visualise an axial distance of 4 µm, compared with 300 µm on VH-IVUS. Third, consensus definitions for IVUS-defined phenotypes have only recently emerged22, although our phenotype classification scheme was based on a histopathologic system developed using sudden cardiac death subjects ranging from early intimal deposition (adaptive intimal thickening) through progressive atherosclerotic plaque phenotypes (pathological intimal thickening, TCFA, fibroatheroma, and fibrocalcific)15. We believe the ongoing PROSPECT trial (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) (http://clinicaltrials.gov #NCT00180466) will establish whether a link exists between TCFA, plaque burden and symptomatic plaque rupture. PROSPECT has fully enrolled 700 patients with acute coronary syndromes undergoing multivessel IVUS and will specifically explore the morphologic and compositional features of rupture-prone plaque. Patients will be followed for up to five years for recurrent events.
Since our analysis consisted of only one vessel per patient, inherent sampling bias cannot be discounted, nor can we comment on plaque burden or composition in other, non-analysed, vessels. Due to the cross-sectional design of this study, we cannot establish a relationship between TCFA, plaque rupture, and complications of coronary atherosclerosis. Evaluation in a study with longitudinal follow-up using both volumetric and compositional measures of coronary plaque is necessary to determine whether increased risk of adverse events can be localised to a segment of a coronary artery.
In the most diseased 10 mm segment of a single coronary artery, VH-IVUS demonstrated greater quantity of plaque and a greater proportion of plaque classified as TCFA in patients with greater Framingham risk scores. These data suggest that patients with CHD have unique plaque characteristics which may be potentially useful in studying the future location and risk of plaque rupture, evaluating novel plaque-modulating therapies, and expanding the biological understanding of human coronary atherosclerosis.
Acknowledgements
We thank Jose Aceituno and Joseph Murphy for publication assistance.
Global VH-IVUS Participating Centers: Munich University Hospital Innenstadt (Munich, Germany): V. Klauss, MD; Herz-und Neurozentrum (Kreuzlingen, Switzerland): M. Pieper, MD; AKH / Vienna General Hospital (Vienna, Austria): D. Glogar, MD; Leiden University Medical Center (Leiden, The Netherlands): M. Schalij, MD; Haukeland University Hospital (Bergen, Norway): O. Bleie, MD; Jagiellonian University Hospital (Krakow, Poland): D. Dudek, MD; Centre Hospitalier Universitaire de Besançon (Besançon, France): F. Schiele, MD; Hospital Clinico San Carlos (Madrid, Spain): F. Alfonso, MD; Thoraxcentrum Erasmus Medical Center (Rotterdam, The Netherlands): P.W. Serruys, MD; Westdeutsches Herzzentrum (Essen, Germany): R. Erbel, MD; O.L. Vrouwziekenhuis (Aalst, Belgium): W. Wijns, MD; Marques de Valdecilla University Hospital (Santander, Spain): J. De la Torre, MD; Cliniche Humanitas Gavazzeni (Bergamo, Italy): P. Sganzerla, MD; Istituto Clinico Humanitas (Rozzano, Italy): P. Presbitero, MD; Tel Aviv Sourasky Medical Center Ichilov Hospital (Tel Aviv, Israel): S. Banai, MD; Rabin Medical Center (Petah Tiqwa, Israel): R. Kornowski, MD; Hospital do Meixoeiro (Vigo, Spain): A. Iniguez, MD; Centre Hospitalier Universitaire Vaudois (Lausanne, Switzerland): E. Eeckhout, MD; Lund University Hospital (Lund, Sweden): J. Harnek, MD; Hospital de Santa Marta (Lisbon, Portugal): R. Ferreira, MD; University of California-Davis Medical Center (Sacramento, CA, USA): J. Rogers, MD; Mid-America Heart Institute (Kansas City, MO, USA): S. Marso, MD; Miami Heart Institute (Miami, FL, USA): J.Margolis, MD; Mayo Clinic (Rochester, MN, USA): A. Lerman, MD; Winchester Medical Center (Winchester, VA, USA): J. Pittaluga, MD; Cleveland Clinic (Cleveland, OH, USA): M. Tuzcu, MD; Arizona Heart Institute (Phoenix, AZ, USA): N. Dib, MD; Pinnacle Health Heart & Vascular Institute (Harrisburg, PA, USA): R. Dave, MD; St. Louis University Hospital (St. Louis, MO, USA): M. Lim, MD; Mt. Clemens General Hospital (Mt. Clemens, MI, USA): S. Kazziha, MD; Forsyth Hospital (Winston-Salem, NC, USA): U. Khawaja, MD; St. Joseph’s Hospital (Atlanta, GA, USA): L. Heller, MD; New York Presbyterian Hospital/Cardiovascular Research Foundation (New York, NY, USA): M. Leon, MD; NY Methodist Hospital (Brooklyn, NY, USA): T. Sacchi, MD; Toyohashi Heart Center (Oyama-cho, Toyohashi, Japan): O. Katoh, MD; Rinku General Medical Center, Izumisano Municipal Hospital (Izumisano, Osaka, Japan): S. Sumitsuji, MD; Nihon University Itabashi Hospital (Itabashi-ku, Tokyo, Japan): S. Saito, MD.

