Abstract
Aims: To investigate whether the use of intravascular ultrasound virtual histology (IVUS-VH) leads to any improvements in stent deployment, when performed in patients considered to have had an optimal percutaneous coronary intervention (PCI) by quantitative coronary angiography (QCA).
Methods and results: After optimal PCI result (residual stenosis by QCA <30%), IVUS-VH was performed in 100 patients by protocol, with the option to use the information left to the discretion of the operators. Patients were categorised as: Group1 (n=54), where the IVUS-VH findings were used to evaluate the need for further optimisation of the stent deployment; and Group2 (n=46), where the IVUS-VH was documentary such that the stenting results were considered optimal according to QCA. Optimal stent deployment on IVUS-VH was defined as: normal stent expansion, absence of stent malapposition, complete lesion coverage as indicated by a plaque burden (PB%) between 30-40% and necrotic core confluent to the lumen <10% or PB%<30% at the 5 mm proximal and distal to the stent. The first IVUS-VH in all patients demonstrated the achievement of optimal stent deployment, incomplete lesion coverage, stent under-expansion and stent-edge dissection in 60%, 31%, 20% and 8% of patients, respectively. There was no stent malapposition. In Group 1, 25 patients had optimal stent deployment and did not require further intervention, whilst in 29 patients further intervention was needed (additional stent, n= 18; post-dilatation, n=29). Overall optimal stent deployment was finally achieved in 52/54 patients (96%) in Group 1 and 35/46 (76%) of Group 2, p<0.05.
Conclusions: IVUS-VH may have a role in facilitating optimal stent implantation and complete lesion coverage.
Background
Achieving optimal stent deployment has become a major issue, especially following the introduction of drug eluting stents (DES). Previous intravascular ultrasound (IVUS) studies have implicated suboptimal stent deployment as a contributing factor in DES restenosis1-3 and thrombosis.4,5 Moreover stent under-expansion can often be observed with IVUS despite a good angiographic result,6 and even after high pressure balloon post-dilatation.7 IVUS guided percutaneous coronary interventions (PCIs) may result in improved clinical outcomes when compared to the conventional angiography guided PCI.8-12
However, these findings have not been sufficient for IVUS guidance during PCI to receive a strong level of recommendation in either the AHA or European Guidelines neither in the AHA or European Guidelines.13,14
Until now, the concept of optimal stent deployment has always been limited to the achievement of optimal stent expansion and complete apposition of stent struts to the vessel wall. Little data is available on the potential utility of IVUS for the attainment of the optimal stent length in order to ensure complete lesion coverage.15
A technique that uses spectral analysis of the radiofrequency ultrasound backscatter signals, known as intravascular ultrasound virtual histology (IVUS-VH), has recently been validated both in vitro and in vivo allowing a detailed in vivo assessment of plaque composition.16-18 This allows the identification of IVUS-VH derived thin-cap fibroatheroma (TCFA), the plaque type that shows the highest association with plaque rupture, as assessed by post-mortem histopathology.19
The aim of this study was to investigate whether the use of IVUS-VH leads to any improvements in stent dimensions and completeness of lesion coverage, when performed in patients considered to have had an optimal PCI as assessed by quantitative coronary angiography (QCA).
Methods
The present study is a sub-study of the Atheroremo trial (European Union Grant, Number 201668).20
The Atheroremo trial is a single centre, prospective observational study that is part of the multicentre European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis. The main objective of the study is to correlate the coronary artery disease imaging phenotype as determined by IVUS-VH of a non-intervened and/or intervened vessel with more than 1/3 of the length of the study vessel available for the examination with biomarkers (genetic profile, lipid profile and endothelial progenitor cells).
The main inclusion criteria were: any patient more than 21-years old, with stable angina pectoris (CCS Class 1, 2, 3 or 4) or unstable angina pectoris (Braunwald Class 1-3, B-C) or patients with documented silent ischaemia or with an acute myocardial infarction (ST- and non-ST elevation myocardial infarction).
In this sub-study any patient meeting the criteria for the Atheroremo study was suitable for inclusion. After the completion of coronary stenting, and the attainment of an optimal angiographic result, which was defined as a residual stenosis <30%,21 IVUS-VH was performed by protocol. A qualitative evaluation of this first documentary IVUS-VH was performed in all patients at the time of the procedure for the presence of malapposition, edge dissection, incomplete lesion coverage and stent under-expansion when compared to the normal reference vessel segment.
A quantitative on-line assessment of the worst site was performed to quantify % plaque burden and % necrotic core. Further intervention at this stage was left to the discretion of the operator and a repeat IVUS-VH was only performed if a further intervention was carried out. At the end of each procedure operators documented whether the findings from the first IVUS-VH had guided the decision to perform further intervention, enabling patients to be categorised into two groups: Group 1, where the IVUS-VH findings were used to evaluate the need for further optimisation of the stent deployment using additional balloon inflations, and/or additional stents; and Group 2, where the IVUS-VH was documentary such that the stenting results were considered optimal according to the QCA and the final decision not to perform further intervention was made prior to and irrespective of the IVUS-VH result (Figure 1).
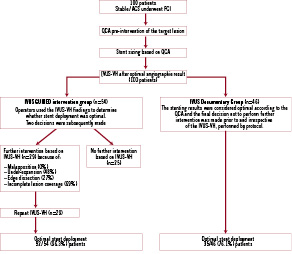
Figure 1. Study design.
The quantitative analysis was performed off-line on the final IVUS-VH in both groups.
Invasive coronary angiography
Coronary angiography was performed according to local practice most frequently via the femoral route. Multiple projections were obtained after intracoronary nitrate injection. The QCA measurements were computed on a still frame at end diastole in the same angiographic view selected by the interventional cardiologist pre- and post-stenting. Percentage diameter stenosis, lesion length, minimal lumen diameter, maximal lumen diameter and interpolated reference vessel diameter were computed before and after the PCI (CAAS II; Pie-Medical, Maastricht, The Netherlands). The initial stent size was determined using the QCA parameters of the lesion, namely: the interpolated reference lumen diameter, the maximal diameter and the obstruction length. The maximal diameter on the diameter function was the determinant factor in the selection of stent diameter (Figure 2).
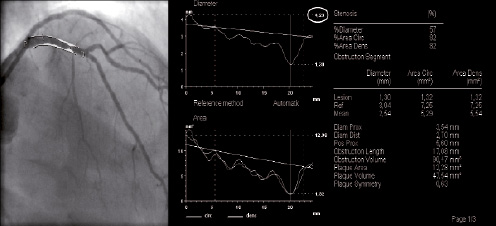
Figure 2. Pre-procedural quantitative coronary angiography for the determination of the stent size. The maximal diameter on the diameter function (white circle) was the determinant factor in the selection of stent diameter.
An optimal angiographic stent deployment was defined as a residual stenosis <30% by QCA.
IVUS-VH imaging
IVUS-VH was performed with a 20MHz catheter (2.9 Fr; Volcano Therapeutics Inc., Rancho Cordova, CA, USA ) at 0.5 mm/sec automatic pullback rate. The data were analysed using the VIAS software (computer-based qualitative and quantitative analysis software; Volcano Corp., Rancho Cordova, CA, USA).
Analytical plan
The analysis of the final IVUS-VH in both groups was performed off-line as follows:
– Stent analysis, in which the region of interest was the stented segment.
The following IVUS parameters were computed: mean stent area, minimal stent area, minimal stent diameter, maximal stent diameter, stent under-expansion index (minimal stent area/average reference lumen area). A normal stent expansion was defined as an under-expansion index ≥0.8.
Malapposition was defined as separation of at least one stent strut from the luminal surface of the arterial wall that was not overlapping a side branch.
– Stent edges analysis, involving a length of 5 mm proximal and 5 mm distal to the stent edges.
In the stent edges analysis tissue characterisation of the plaque, described as a percentage of plaque area (fibrous tissue %, fibro-fatty %, dense calcium %, necrotic core %), its extension ([plaque burden: vessel area- lumen area/vessel area]x100) were computed.
The reference area was defined as the site with the least plaque burden within the 5 mm proximal and distal to the stent edges. The average between the proximal and the distal reference measurements was computed.
Incomplete lesion coverage was considered present when the plaque burden at the 5 mm distal and proximal to the stent was between 30-40% and the necrotic core was >10% or the plaque burden was >40%, irrespective of the necrotic core %.
Morphological patterns
The morphological classification of the plaque was performed at the worst site (highest plaque burden %) according to the modified American Heart Association histological classification by Virmani et al19,22 adapted for IVUS-VH plaque classification based on plaque composition and location of different tissue components, as listed below:
– Pathological intimal thickening (PIT): ≥600 µm thickness for >20% of the circumference with fibro-fatty tissue >15%, and no confluent necrotic core or dense calcium.
– Fibroatheroma (FA): Dominant fibrotic tissue and confluent necrotic core or dense calcium >10%, not in contact with the lumen.
– Fibroatheroma with previous healed ruptures: multiple layers of necrotic core roofed by dense calcium, fibrous and/or fibro-fatty tissue.
– Thin cap fibroatheroma (TCFA): confluent necrotic core component >10% abutting the lumen without evidence of a fibrotic cap, dense calcium >5%, plaque burden >40%.
– Fibrocalcific plaque: mainly fibrous plaques, with confluent dense calcium >10% and no confluent necrotic core.
Definition of optimal stent deployment by IVUS-VH
Optimal stent deployment by IVUS-VH was defined when all of the following criteria were satisfied:
– Normal stent expansion.
– Absence of stent malapposition.
– Plaque burden between 30-40% and necrotic core confluent to the lumen <10% at the proximal and distal edges or a plaque burden <30% at the proximal and distal stent edges.
Statistical analysis
Continuous variables were presented as mean±SD. Categorical data were expressed as counts and percentages. Continuous variables were compared by 2-tailed student t test. The Pearson χ2 test was used for comparing frequency of occurrence. A probability value of <0.05 was considered significant.
Results
The baseline characteristics of the 100 patients in this sub-study are displayed in Table 1.
Optimal angiographic stent deployment was achieved in all patients.
The first IVUS-VH pullback demonstrated optimal stent deployment in 60 (60%) patients, together with incomplete lesion coverage in 31/100 patients, stent-edge dissection in 8/100 patients, and stent under-expansion in 20/100 patients. There was no stent malapposition.
After the first IVUS-VH, 54 patients were categorised into Group 1 and 46 patients into Group 2. Further intervention was carried out in 29 patients in Group 1 (additional stent, n=18; post-dilatation, n=29), whilst the remaining patients in Group 1 (n=25) were deemed not to require additional intervention after reviewing the IVUS-VH. After further intervention optimal stent deployment was finally achieved in 52/54 patients (96%) in Group 1 and 35/46 (76%) of Group 2, p<0.05 (Figure 1).
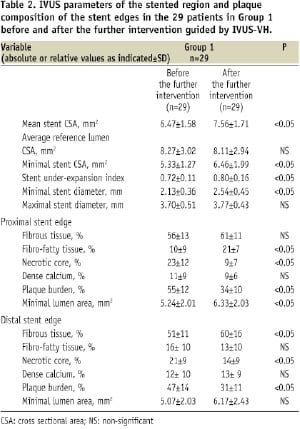
Table 2 demonstrates the significant improvements in the stent dimensions and lesion coverage that were observed in the 29 patients of Group 1 as a result of the additional IVUS-guided intervention.
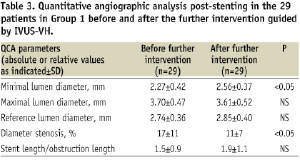
Similarly Table 3 shows the significant improvements in the QCA parameters, minimal lumen diameter and residual percent diameter stenosis in these 29 patients.
The final IVUS findings in the stented region, the tissue plaque composition, the plaque burden and the minimal lumen area at the proximal and distal stent edges are described in Table 4.
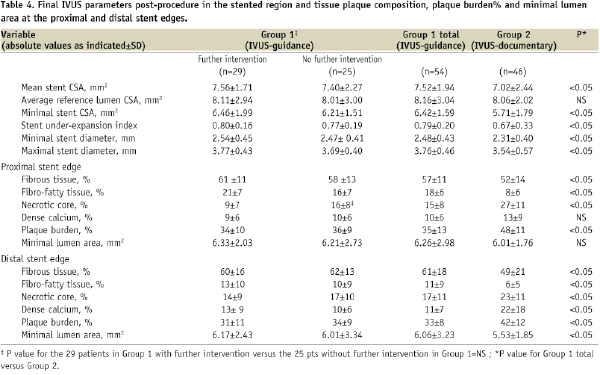
The mean stent area, the stent under-expansion index and the minimal stent area were significantly higher in Group 1. The plaque burden and the necrotic core component were significantly higher in Group 2 in both proximal and distal edges. Incomplete lesion coverage within the 5 mm proximal to the stent was observed in 2/54 (4%) patients in Group 1 and in 11/46 (24%) patients in Group 2, p<0.05.
The IVUS-VH plaque types at the proximal stent edge in Group 1 were: fibro-calcific plaques, n=2; and Group 2 were fibroatheroma, n=3; fibroatheroma with previous healed ruptures, n=5; TCFA, n=3. At a distance of 5 mm distal to the stent there was no incomplete lesion coverage observed in Group 1, whilst 7/46 (13%) patients showed incomplete lesion coverage in Group 2, p<0.05. The plaque types at the distal stent edge in the Group 2 were: fibroatheroma, n=2; fibroatheroma with previous healed ruptures, n=3; and TCFA, n=2.
The pre-procedural and post-procedural QCA are displayed in Table 5.
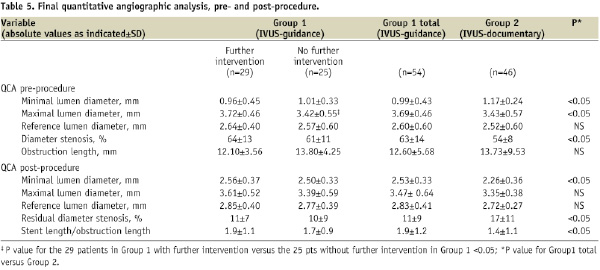
The QCA post-stenting showed that the reference lumen diameter was not significantly different between the two groups (2.83±0.41 mm vs. 2.72±0.27 mm, p=NS), whilst the minimal lumen diameter post-stenting achieved in Group 1 was significantly higher when compared to Group 2 (2.53±0.33 mm vs. 2.26±0.36 mm, p<0.05). The number of implanted stents/lesion was 1.6±0.7 in Group 1 versus 1.2±0.5 in Group 2, p<0.05. The mean stent length was 23.94±6.20 mm in Group 1 versus 19.22±10.48 mm in Group 2, p<0.05. The maximum balloon pressure was 17.6±2.5 atm in Group 1 and 16.4±3.1 atm in Group 2, p<0.05.
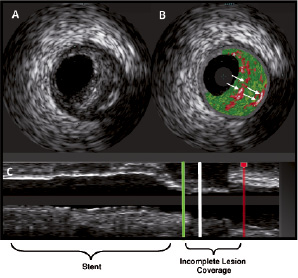
Figure 3. Documentary IVUS showing a cross-sectional IVUS grey-scale (A) and virtual histology (B) of an incomplete lesion coverage at the proximal stent edge. The longitudinal pullback (C) shows the region of incomplete lesion coverage proximal to the implanted stent (within the green and red lines) and the corresponding frame of the cross-sectional images (white line). The virtual histology image (B) shows a fibroatheroma with previous healed ruptures (arrows).
Discussion
The main finding of this study is that IVUS-VH analysis is a useful tool for the identification and tissue characterisation of incomplete lesion coverage after stent implantation, in patients deemed to have an optimal procedure based on QCA (residual stenosis<30%).
In this study a new definition of optimal stent deployment was adopted in order to evaluate either the attainment of an optimal stent diameter, or complete lesion coverage according to IVUS-VH criteria.
Previous studies have focused on IVUS gray scale guidance during stent implantation for the achievement of an optimal stent cross-sectional area, and the general conclusions have always been, as expected, that larger stent areas are associated with a better acute and long-term outcome.8-12 Little data however is available on the assessment of the stent edges using IVUS to determine whether full lesion coverage has been achieved, and whether this was ultimately beneficial for the patient.15
Of note, the initial IVUS-VH findings showed that an optimal stent deployment was achieved in only 60% of the patients, despite all them being considered to have had an optimal angiographic result from stenting. The advantage of using this IVUS-VH to guide further intervention, which was discretionary in this study, is highlighted by the significantly greater optimal stent deployment achieved in those patients in Group 1 compared to Group 2.
The only reason optimal stent deployment was not achieved in all Group 1 patients was because two patients had a fibro-calcific plaque observed at the proximal stent edge, which was located at the ostium of the left anterior descending and circumflex artery, respectively. The plaque burden of these both lesions was >40%, however in both cases the MLA was >4.0 mm2, which has been shown to be associated with a low rate of MACE.23 In this case, after functional evaluation, the operator decided to defer implantation of an additional stent, which would have ultimately landed in the distal left main stem.
IVUS-VH analysis identified the presence of vulnerable plaques (IVUS-VH TCFA) in 5/46 (11%) patients within 5 mm proximal and distal to the stent.
In the present study plaque characterisation was performed 5 mm from the stent edges, after an optimal angiographic stent deployment. Nevertheless, 24% of the patients in Group 2 and 4% in Group 1 showed a plaque burden >40% at these sites. In Group 2 the rate of IVUS-VH TCFA was 11%, whilst 17% of these patients had fibroatheromas with previous healed ruptures.
Histopathology studies have shown that plaque rupture is the suspected cause of death in 60% of patients with sudden coronary death and thrombosis, and of these patients, 75% show previous sites of plaque rupture.24 Lesions with healed ruptures may exhibit multi-layering of lipid and necrotic core, suggestive of previous episodes of thrombosis.25
Recently, the PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) randomised trial has shown that the presence of IVUS-VH TCFA is a significant predictor of major adverse cardiac events (MACE) during three years of follow-up (HR: 3.00 [1.68, 5.37]; p=0.0002). The prospective identification by IVUS-VH of lesions prone to develop MACE has been enhanced for the first time in this randomised study that enrolled 700 patients with acute coronary syndromes in whom QCA and IVUS-VH were performed in the entire coronary tree. The IVUS-VH plaque characterisation in the PROSPECT study revealed that 51.2% of patients had ≥1 VH-TCFA with an average number of IVUS-VH TCFAs per patient equal to 0.97±1.30 (range: 0-7 per patient).26
At present IVUS-VH represents a useful modality for the characterisation of clinically relevant plaque components. However the findings of this study, after the recent results from the PROSPECT trial, raise an important issue: do we always need to extend lesion coverage when a plaque burden >40% and a necrotic core confluent to the lumen >10% at the stent edges are detected, although the angiographic result is optimal?
This question has no easy answer. Whilst on one hand, the presence of IVUS-VH TCFAs has been shown to be a predictive factor in major cardiovascular events, the perceived treatment of these TCFAs is not straight forward. The deployment of multiple overlapping stents, even in the DES era, is still associated with an increased risk of MACE.27-29 In addition, the length and number of DESs implanted is associated with an increased risk of stent thrombosis.30-32
Further complicating the picture is evidence indicating that the implantation of balloon-expandable stents into vulnerable lesions can lead to plaque prolapse and luminal thrombosis.33,34 This is related to the high radial force caused by high-pressure balloon inflation and penetration of stent struts into the necrotic core. Ideally, lesion coverage and cap reinforcement by a dedicated shield with struts strong enough to progressively change the geometry of the lumen by self-expansion without rupturing the cap would be appealing for the prevention of vulnerable plaque rupture, as indicated by the 6-month follow-up of the SECRITT study.35
Targeted medical therapy is a valid option. The ASTEROID36 study demonstrated a significant regression of the atherosclerotic plaque burden in coronary disease patients treated with rosuvastatin 40 mg/dl. This study reported regression of atheroma volume in 78% of patients, but it is unknown whether these changes in plaque size were associated with modification of plaque composition. The on-going IBIS-3 trial has been designed to evaluate the changes in necrotic core by IVUS-VH and near-infrared spectroscopy in patients treated with rosuvastatin 40 mg/dl in order to address this unanswered question.
Study limitations
The present study is limited by the small sample size and by the lack of clinical and angiographic follow-up. However, it was only designed to provide preliminary observations and to generate hypotheses for future studies. Additionally the study is hampered by the lack of prospective randomisation; the choice whether to use the IVUS findings to evaluate the need for further optimisation of the stent deployment was left to the discretion of the operators and therefore subject to inter-operator variability.
The 0% rate malapposition that was observed in this population deserves additional consideration as this is lower than has been observed in previous studies,37 where rates of postprocedural malapposition have ranged from 2.6%-22%. This could be explained by a possible selection bias and/or a consequence of the low sensitivity of the 20 MHz IVUS catheter for the visualisation of at least one strut that is clearly not in contact with the vessel wall. Moreover, it must be acknowledged that the initial stent size was always determined by on-line pre-stenting QCA. The stent diameter was chosen based on the maximal diameter measured by the QCA, which could have contributed to reduce the occurrence of malapposition.
Conclusions
IVUS-VH guidance can facilitate stent implantation with the attainment of optimal stent expansion and lesion coverage. However, future randomised trials are needed to demonstrate if any improvement in clinical outcome occurs as a result of optimal stent implantation as defined by IVUS-VH criteria compared with the classical angiographic definition.
Acknowledgements
We would like to thank Elco van den Heyden, Bsc, for his careful contribution in retrieving the clinical data and performing the QCA analysis of these patients.

