Abstract
In current cardiology practice, many patients undergo secondary revascularisation due to reduced long-term vein graft patency or in-stent restenosis. In this report, we describe causes of drug-eluting stent restenosis identifiable by intravascular ultrasound imaging (IVUS) and variables related to restenosis used for reporting greyscale IVUS. In addition, IVUS findings in bypass grafts and the long-term results after stent implantation are provided. Finally, the usefulness of IVUS Virtual Histology for the study of restenosis is described.
Introduction
Although coronary artery bypass grafting (CABG) is the preferred treatment modality for multivessel coronary artery disease or left main coronary disease,1 its effectiveness is limited by reduced long-term vein graft patency. The alternative revascularisation approach is percutaneous coronary intervention (PCI), but has worse 1-year clinical events compared to CABG, mainly due to restenosis. As a result, many patients undergo secondary revascularisation.
Stenting has been highly effective in reducing the development of restenosis when compared with percutaneous coronary angioplasty2. The advent of drug eluting stents (DES) has reduced this complication further3. Conventional invasive coronary angiography is the most commonly used tool to detect in-stent restenosis. Quantitative coronary angiographic (QCA) measures to assess in-stent restenosis can include late luminal loss, temporal changes in diameter stenosis, and binary restenosis4. Although late loss is highly related to the risk of coronary revascularisation in stent trials, this is only an indirect measure for intimal hyperplasia. Intravascular ultrasound (IVUS) can directly detect and quantify the presence of tissue at the adluminal side of the stent (i.e. intimal hyperplasia) which has been associated with adverse clinical events5.
Complementary to greyscale IVUS, tissue characterisation by IVUS radiofrequency data (RFD, or Virtual Histology [VH]) analysis has the potential to add valuable information on the pathogenesis of restenosis by providing information on plaque composition. IVUS-VH analysis is a relatively new technique and its clinical usefulness in this context has still to be elucidated.
Drug-eluting stent use has been extended considerably, currently many patients that undergo secondary revascularisation, that is treatment of bypass grafts after CABG or patients with bare-metal/drug-eluting in-stent restenosis, are treated with these devices. Specially in saphenous vein grafts (SVG), the beneficial effects of stenting are hindered by a high incidence of procedural complications such as thrombosis, distal embolisation, and high re-occlusion rates compared to patients treated for native vessel disease. Therefore, it has been suggested that periprocedural IVUS assessment would improve long-term results following stent implantation, especially by giving some light into the plaque morphology and sizing of the stent in order to avoid plaque dislodgement and distal embolisation. While in patients with restenotic lesions, both sirolimus- and paclitaxel-eluting stents have shown superiority to balloon angioplasty and brachytherapy6. In these latter studies, the use of IVUS was crucial to better understand the mechanisms associated with the better outcome.
In this report, the following aspects of intracoronary ultrasound and stent assessment are reviewed: 1. causes of drug-eluting stent restenosis identifiable by IVUS (i.e. stent under-expansion and stent fracture); 2. critical appraisal of the variables used for reporting greyscale IVUS findings related to restenosis in drug eluting stent studies (e.g. percentage intimal hyperplasia volume); 3. insights into the study of restenosis in bypass grafts after long-term stent implantation; and 4. usefulness of IVUS Virtual Histology for the study of restenosis.
Causes of drug-eluting stent restenosis identifiable by IVUS
The MUSIC study7 evaluated the impact of IVUS-guided stent implantation on the 6-month restenosis rate. In this study, three main IVUS variables were considered for assessing optimal stent deployment: complete stent apposition, well expansion (i.e., in-stent minimal lumen area >90% of the average reference lumen area) and symmetric stent expansion defined as minimum lumen diameter divided by maximum lumen diameter ≥0.7. When IVUS showed optimal stent expansion, satisfactory clinical and angiographic outcome at six months were achieved. This study highlights that appropriate evaluation of stent deployment by IVUS (i.e. complete and symmetric stent expansion) impact restenosis rate.
In a substudy of SIRIUS8, the impact of post-stenting sirolimus-eluting and bare metal stent (BMS) dimensions on long-term patency was assessed by intravascular ultrasound analysis. The receiver operating characteristic curve identified post-stenting minimum stent area values that predicted adequate follow-up minimum luminal area (defined as minimum luminal CSA >4 mm2): 5 mm2 for SES and 6.5 mm2 for BMS.8 This study suggests again that stent under-expansion plays an important role in stent failure. In another sirolimus-eluting stent study that included 550 patients (670 lesions) with 6-month follow-up angiography, the highest restenosis rate was observed in lesions with stent area <5.5 mm2 and stent length >40 mm.9
Unfortunately, optimal post-stenting minimum stent area values are not frequently achieved in current practice. In a study that enrolled 200 patients with de novo coronary lesions treated with either sirolimus-eluting stent (n=133 patients) or paclitaxel-eluting stent (n=67 patients) stent under IVUS guidance, 24% of sirolimus-eluting stent and 28% of paclitaxel-eluting stent did not achieve a final MSA of 5 mm2.10 In a more recent randomised study that included only diabetic patients, 42.5% vs. 10.5% (p=0.002) of the Cypher Select and Taxus Express-2 stents, respectively, did not achieve a final minimum stent area of 5 mm2.11
Another reported cause of stent restenosis is stent fracture. At two US centres, 3,920 patients in a period of one year were treated with DES, in-stent restenosis was reported in 188 patients (121 and 67 sirolimus- and paclitaxel-eluting stents respectively). In 35 patients out of 188 (18.6%), stent fracture was observed. Only cases with severe stent (complete separation of stent segments) were included. Thus, it appears that in one-fifth of the cases of in-stent restenosis, stent fracture is the main mechanism of restenosis. The presence of vessel tortuosity, use of sirolimus-eluting stents and overlapping stents were the factors more frequently associated with stent fracture, while larger stent diameter was protective. According to the authors of this study, high magnification cine-angiography is the best method to diagnose stent fracture and, although intravascular ultrasound was performed in 10 out of the 35 cases of stent fracture, it did not add any value to high magnification cine-angiography.12
Common definitions used in reporting IVUS stent studies (Figures 1-3)
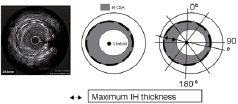
Figure 1. Once the lumen and the stent are traced, the software detects the centroid of the stent and constructs 180 lines through this centroid. The computer calculates the maximal distance between the lumen border and stent, the maximum IH thickness. Reproduced with permission from Elsevier. From Escolar E, et al. Am J Cardiol. 2007;100(4):621-626.
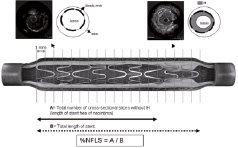
Figure 2. The number of cross-sectional image slices (1 mm apart) with no detectable neointimal hyperplasia using IVUS is divided by total length of the stent. EEM, external elastic membrane; IH, intimal hyperplasia. Reproduce with permission from Elsevier. From Escolar E, et al. Am J Cardiol. 2007;100(4):621-626.
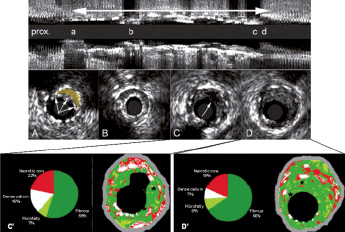
Figure 3. Top: longitudinal view of the greyscale IVUS. The double-headed arrow shows the stent location. Letters “a” through “d” indicate position of the cross-sectional panels “A” through “D”. Panel A, shows stent malapossiton (arrows pointing at stent struts), in yellow the malappossed area. Panel B, shows the minimum luminal area in the stent (4.6 mm2). Panel C, depicts neointimal hyperplasia within the stent in which a rupture has occurred. Panel C’ is the corresponding IVUS VH image shown in panel C. Of note, the stent struts in the IVUS VH image appear as dense calcium surrounded by necrotic core (*). In panel D an intimal hyperplasia with intact luminal surface is shown. In panel D’ the corresponding IVUS VH image of panel D, again the stent struts appear as dense calcium and necrotic core, while the intimal hyperplasia is characterised as fibrous and fibrofatty tissue.
Intimal hyperplasia area (IH, mm2), defined as stent area minus lumen area.
Percentage of intimal hyperplasia (% IH), defined as stent area divided by lumen area.
Maximum percentage of intimal hyperplasia, defined as the largest percentage of intimal hyperplasia within the length of the stent. Maximum of neointimal area/stent area X 100.
Percentage volume of intimal hyperplasia (%vol IH), defined as IH volume divided by stent volume.
IVUS binary restenosis, defined as maximum percentage of IH greater than 50%.
Maximal intimal hyperplasia thickness (mm), defined as the longest distance between the lumen and stent countours, considering the center of mass of the stent.
Percentage of neointimal free length of stent (%NFLS), defined as the stent length with no detectable neointimal hyperplasia using IVUS divided by total length of the stent.13
Greyscale IVUS findings related to restenosis in drug eluting stent studies
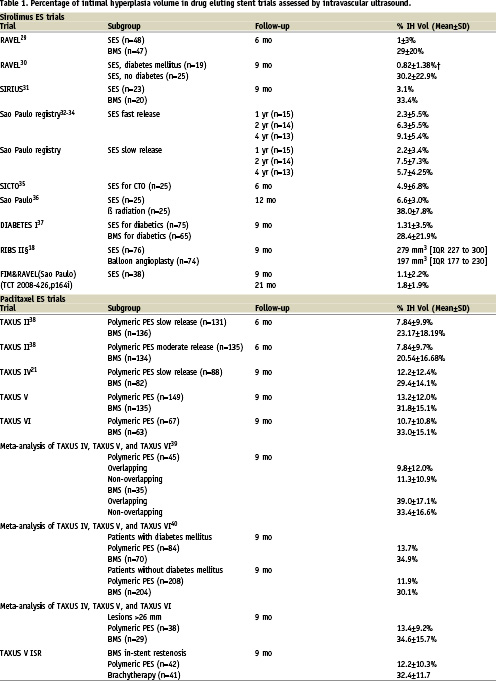
It is clear that DES reduces intimal growth as compared to BMS (Table 1). This effect can vary depending on the type of eluted drug, patient and lesion subsets. Currently, there are no standard guidelines for the reporting of IVUS in stent studies. As a result, there is heterogeneity in describing IVUS results in the literature. Here we discuss the rationale of some variables and their potential clinical implications.
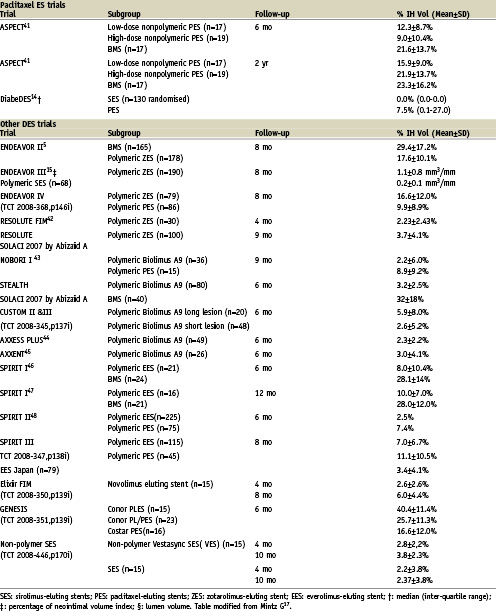
Previous studies have shown that the pattern of restenosis associated with DES is a focal one. We believe, therefore, that an important parameter to examine is the maximum intimal hyperplasia. This parameter has been found to correlate moderately with angiographic late loss (r values can range 0.47 to 0.52)13; highlighting a limitation of detecting restenosis using angiographic late loss. The percentage of neointimal free length of stent (%NFLS) provides useful information on the longitudinal distribution of the neointimal hyperplasia. A meta-analysis of TAXUS IV, V and VI demonstrated that nearly half of the stent length was free of IH in the Taxus group (48.8±36.0% vs. 13.4±22.1% in the control group, p <0.0001)13. In another study comparing the paclitaxel stent and sirolimus stent, 46.1 vs. 5.4% of the stent length had covering, p < 0.001, respectively14. For the zotarolimus-eluting stent similar intimal hyperplasia distribution (50.2% of the stent length had coverage) as the Taxus stent has been reported15. It has been suggested that the “patchy” distribution of intimal growth associated with DES (i.e., lack of neointimal tissue in the mid-portion of the stent) could be related to a larger concentration of drug in that portion of the stent16.
One of the most common variables used to report restenosis is percentage intimal hyperplasia volume in the stent segment17 (Table 1). This variable normalises the intimal hyperplasia to the stent length therefore allowing the comparison of different stent types (BMS vs. DES), as well as different drug types (i.e., SES vs. PES). The percentage intimal hyperplasia, however, minimises the impact of focal restenosis. For example, a flow-limiting focal stenosis in a long stented region may provoke clinical symptoms but the calculated percentage of intimal hyperplasia will be small. Indeed, in a report that explored the association between percentage intimal hyperplasia volume and maximum percentage of intimal hyperplasia with target lesion revascularisation, the sensitivity and specificity were better for the latter parameter (30% of percentage neointimal volume had a sensitivity of 70% and a specificity of 77.3% and 55% of maximum percentage neointimal area had a sensitivity of 80.0% and a specificity of 82.6%)5.
Thus, while reporting IVUS studies, it is important to provide the reader with as much information as possible, including clinically relevant variables and also variables that allow comparison between devices.
IVUS results of patients treated for secondary revascularisation: patients with in-stent restenosis
In the RIBS-II trial,18 a comparison between balloon angioplasty (n=74) and sirolimus-eluting stent (n=76) in patients suffering from in-stent restenosis was reported. The primary endpoint (i.e. restenosis rate), showed a marked difference between balloon angioplasty and sirolimus-eluting stent (11 vs. 39%, p <0.001). Secondary IVUS endpoints were lumen and neointima proliferation volume at nine months. IVUS studies were available in 40 in the balloon group and 42 in the sirolimus group. Lumen volume was larger in the SES group (279 mm3 vs. 197 mm3; p < 0.001). Authors mentioned that the main factor contributing to larger lumen volume in the sirolimus-eluting stent group was the important inhibition of intima hyperplasia.
Edge effects assessed by greyscale IVUS
The serial geometrical changes that can occur at the edges of the stent vary depending on the location (proximal vs. distal edge), the shear stress conditions19, the morphological baseline characteristics of the segment (degree of obstruction), type of eluted drug and baseline tissue composition.
In TAXUS II20, positive remodelling was observed only at the distal edges in both the slow and moderate release Taxus groups. This was almost entirely due to an increase in plaque size and there was hardly any changes in lumen size. Meanwhile, in the TAXUS IV study21, almost no change was observed in vessel size at the proximal edge and a trend towards negative remodelling was observed at the distal edge. It is difficult to put these two studies into perspective; perhaps due to differences in sample size and potential selection bias acknowledged by the authors of TAXUS IV.
In the ENDEAVOR II study5, IVUS analysis at eight months found the minimum luminal area in the distal edge of the ZES to be significantly larger than that of the BMS. In detailed sub-segmental analyses, loss of the mean luminal area did not differ between the two stent groups at the proximal edges. Conversely, at the first millimetre of the distal edge, the mean plaque area increased largely with the BMS than the ZES, while the mean vessel area was not different between the two stent groups. This resulted in a significantly smaller mean luminal area in the ZES than the BMS group.
Restenosis in bypass grafts after long-term stent implantation
The secondary revascularisation of diseased bypass grafts has been a challenge for both interventionists and cardiac surgeons. The SVGs treated with PCI within one year of surgical implantation have more frequently ostial/proximal, usually focal, disease and have smaller proximal and distal reference lumen areas and greater reference plaque burden than those SVGs treated beyond one year of surgical implantation.22 Few studies have been reported evaluating therapeutical options for this high-risk lesion subset. The SECURE study included 76 patients (n=94 lesions) with graft disease treated with sirolimus eluting stent and were compared with the outcomes of 176 patients (n=311 lesions) with only native vessels. In 14 patients with graft disease, IVUS follow-up was performed at eight months. In-hospital clinical events were similar between groups. Overall, percentage intimal hyperplasia was 11.8±16.5% and half of patients with graft sirolimus-eluting stent had < 1% intimal hyperplasia.23 In the setting of a randomised trial, 75 patients with graft disease (96 lesions) that received either sirolimus-eluting stents or bare metal stent (RRISC study) were assessed by IVUS at six months. Sirolimus-eluting stents showed smaller neointimal hyperplasia volume compared with BMSs (1.3 vs. 24.5 mm3, p<0.001). In sirolimus-eluting stent group, there was a greater intimal hyperplasia at overlapping sites as compared to non-overlapping segments.24
IVUS virtual histology for the study of restenosis
Regarding the vascular responses after stenting (i.e. restenosis and remodelling), a response-to-injury pattern of wound healing occurs, mainly characterised by an increase in smooth muscle cells and extracellular matrix25.
This depends, though, on the drug type and the release kinetics. The most commonly use DES, sirolimus- and paclitaxel- eluting stents reduce restenosis by interfering with muscle cell proliferation through different modes of action16. Imaging techniques that assess tissue composition may help to characterise intima hyperplasia composition, so a better understanding of this process can be achieved.
While using IVUS-VH to assess in-stent restenosis, distinction should be made between metal stents and bioabsorbable stents. The use of IVUS-VH to assess in-stent restenosis in metal stents should be done taking into account the following: lack of validation of the technique in this context, misclassification of the stent struts as “dense calcium” surrounded by necrotic core and the potential interference of the superficial stent struts on the backscattering of the tissue behind them. On the contrary, IVUS-VH can be used in bioabsorbable stents to evaluate serial changes in plaque type and composition by assessing the vessel wall before stent implantation and after fully absorption of the stent. In addition, virtual histology has proved to be useful to follow-up absorption of the polymeric struts.
Recently, the feasibility and safety of a bioabsorbable everolimus-eluting stent (BVS) was assessed. In a prospective, open-label study, 30 patients with a single de novo lesion that was suitable for treatment with a single BVS, were enrolled. Stent struts are classified as “dense calcium” (DC) and “necrotic core” (NC) by IVUS-VH.
From pre- to post-stenting, with VH (n=13), there was an increase in mean “DC” (9.8 vs. 25.4%, p=0.0002) and “NC” (15.5 vs. 30.5%, p=0.0002). Comparing post-stenting with follow-up (n=27), VH showed a decrease in “DC” (29.7% vs. 21.1%, p=0.0001). “NC” also decreased (26.9 vs. 21.5%, p=0.0027). IVUS-VH changes at six months suggest alteration of the BVS with reduction of RF backscattering by polymeric struts.26
Edge effects assessed by IVUS-VH
Based on previous pathological studies, we hypothesised that the tissue associated with increase of plaque at the edges of the paclitaxel stent is mainly fibrofatty tissue as assessed by IVUS-VH. Fibro-fatty, in IVUS-VH, has been described as loosely packed bundles of collagen fibres with regions of lipid deposition and extracellular matrix without necrotic areas27.
In the BETAX study28, 24 patients (26 paclitaxel eluting stents) were studied. Serial expansive vascular remodelling was observed at the proximal and distal edges of the stent to accommodate fibrofatty and fibrous tissue growth. In the first 2 mm adjacent to the proximal edge of the stent, the vessel wall grew to compensate the plaque growth without affecting the lumen size. In the following 3 mm, overcompensation (vessel wall increased more than plaque size) was observed. Consequently, the lumen size increased. At the distal edge, overcompensation was observed in all 5 mm, followed by an increase in lumen size. In summary, proximal and distal growth patterns were characterised by an increase in fibrofatty tissue (p<0.001 and p<0.001, respectively), decrease in necrotic core (p=0.014 and p<0.001, respectively), and decrease in dense calcium content (p<0.001 and p<0.001 respectively).
Conclusions
IVUS greyscale has proved its utility to provide information on restenosis that can be used for clinical and investigational purposes. While IVUS-VH, though is in its infancy stages of development, it can add complementary information to study the edge effects in metal stents and can give insights into the temporal compositional changes in bioabsorbable stent studies.

