Description
Intravascular ultrasound (IVUS) with radiofrequency data analysis is a novel technology which allows identification of atherosclerotic plaque components1-4. Todays, tissue characterisation can be achieved using two different IVUS systems: Virtual Histology™ IVUS (VH, 20 MHz, phased-array transducer, 2.9 F Eagle Eye™ Gold, Volcano Therapeutics, Rancho Cordova, CA, USA), and iMap™ IVUS (iMap, 40 MHz, mechanical-type transducer, 3.2 F Atlantis, Boston Scientific Corporation, Natick, MA, USA). These two methodological approaches so far had not been compared in vivo. The purpose of this paper is to compare overall in vivo findings between these two IVUS radiofrequency based tissue characterisation systems.
Technical specification
By design, these two IVUS catheters have different capabilities for tissue characterisation. Unlike VH, iMap uses a 40 MHz single rotational transducer on a drive shaft and can acquire radiofrequency data continuously, while VH uses an electronic catheter that acquires only ECG gated data. In addition, VH feeds the spectra that are obtained from the radiofrequency data using autoregressive models into a classification tree that has reported diagnostic accuracies of over 90% each plaque components as compared to histology5, while iMap uses a pattern recognition algorithm on the spectra that were obtained from a fast Fourier transformation and a histology-derived database6. Taken together, it is possible that these distinctive features may lead to differences in the tissue characteristics of the images of coronary plaque obtained. iMap and VH (40 MHz vs. 20 MHz) have relative advantages and disadvantages in displaying greyscale images. iMap has higher resolution, but displays specific artefacts such as non-uniform rotational distortion because it is a rotational catheter. In addition, far-field imaging can be more problematic with high frequency catheters due to amplified attenuation and enhanced blood backscatter.
It is well known that mechanical catheters (i.e., iMap) have increased acoustic power since they send all their energy in the same direction. Conversely, the phased-array catheters of VH send this energy in multiple directions. Although the latter improves the resolution, it is a noisy process. Accordingly, this could potentially be the source for some differences7. The colour code for tissue types is different. iMap depicts fibrotic (light green), lipidic (yellow), necrotic (pink) and calcified tissue (blue), while VH depicts fibrous (green), fibrofatty (yellow green), necrotic core (red) and dense calcium (white), respectively.
Indications for use
Tissue characterisation will help us to better understand the intricate process of atherosclerosis. The information we received has served to understand the natural history of coronary artery disease that may ultimately translate into a meaningful treatment for our patients.
Clinical experience
Between January 29, 2009, and March 20, 2009, a total of 23 consecutive patients underwent iMap imaging at the Thoraxcenter, Erasmus MC, The Netherlands, and a total of 30 diseased coronary arteries and 61 pullbacks were performed. The studied vessel was the left anterior descending artery in 15 patients (50%), the left circumflex artery in six (20%) and the right coronary artery in nine (30%). Furthermore, six of those pullbacks were performed without wire because of a wire artefact in the iMap. Out of 23 patients, in 13 patients we performed VH in 16 diseased coronary arteries. All procedures were performed uneventfully.
Various image findings
Imaging of far-field plaque
Although the 40-MHz iMap has a higher resolution than the 20-MHz VH, the image in the 40-MHz catheter is not always clear in the far field and it is also difficult to find the external elastic membrane in large vessels (Figure 1A).
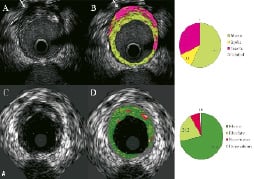
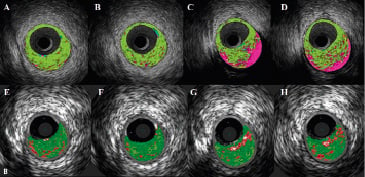
Figure 1. A) Corresponding cross-sections of iMap (top) and VH images (bottom). (A) Conventional IVUS image using a 40-MHz mechanical rotating catheter. Arrow shows that the far-field image cannot be clearer than that obtained with the 20-MHz phased-array catheter (C). Of note, this attenuated area is represented as necrotic core in iMap (B). iMap depicts fibrotic (light green), lipidic (yellow), necrotic (pink) and calcified tissue (blue), while VH depicts fibrous (green), fibrofatty (yellow green), necrotic core (red) and dense calcium (white), respectively.
Figure 1. B) Corresponding cross-sections of each IVUS image. Tissue characterisation is rather similar in plaque types with no severe calcification (A, B vs. E, F respectively), but a much larger amount of necrotic tissue is coloured in calcified lesions and in the far-field plaque (C, D vs. G, H respectively). Thin plaque, including external elastic membrane, is shown as a grey medial stripe in VH (E-H), while iMap shows plaque composition of that area (A-D).
Imaging of lesion type
In calcium-sparing plaques, iMap and VH correlate well; that is, both showed the same layout of the different tissue types. In contrast, in heavily calcified plaques, iMap shows large amounts of necrotic tissue and VH fibrous or fibrofatty because of the acoustic shadowing (Figure 1B).
Imaging of calcium shadowing
Although calcium is an important part of the atherosclerotic plaque, and IVUS has a high sensitivity for its detection, almost all IVUS energy is reflected at the front of the calcium, causing a characteristic signal drop-out or multiple reflections of concentric arcs behind the calcified tissue.
Acoustic shadowing is expressed as fibrous or fibrofatty by VH, while the same regions are characterised as necrotic tissue by iMap (Figures 2A).
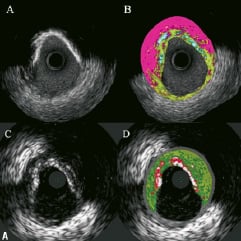
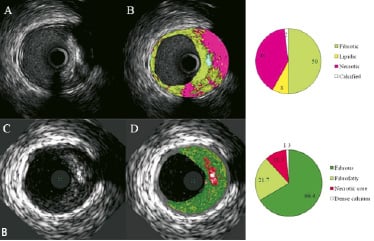
Figure 2. A. Corresponding cross-sections of iMap (top) and VH images (bottom). In panel B, iMap shows large amounts of necrotic tissue behind calcium, while with VH the same area is reported as fibrous or fibrofatty tissue. B. Corresponding cross-sections of iMap (top) and VH images (bottom). iMap shows large amounts of necrotic tissue because of the wire artefact and calcium shadow.
Because reflection of most of the sound leads to no, or little, radiofrequency signals behind the dense calcium, plaque composition behind dense calcified area remains uncertain with both IVUS types. Both algorithms would benefit from identification of regions where the signal to noise ratio is too low, as in – or behind – calcific plaque, so that they could recognise that the algorithm fails.
Also, iMap has a first order attenuation correction, while VH does not. iMap is capable of identifying calcium separately from other tissue types. VH always shows a necrotic core halo around calcium. This has to do with inherent properties of the classification tree.
Imaging of guidewire artefact
Using the monorail catheter technology, the guidewire lies outside the transducer and causes a characteristic artefact, which is distinguished by a narrow-angled shadow.8 The guidewire artefact is expressed as necrotic core tissue with iMap, just like the far field plaque and the acoustic shadowing of the calcium (Figure 2B).
In one case example, in a stented segment, VH-estimated necrotic core volume was 28.8 mm3 and 28.5 %, while iMap showed much larger necrotic core (67.1 mm3 and 44 %) because of the presence of the guidewire artefact.
Imaging of thin plaque
When the plaque is less than the thickness of the artificial grey medial stripe in VH (<250 microns), no plaque composition is given. However, iMap can show plaque composition without imposing any medial area (Figure 1B).
Imaging of stent findings
Currently, none of the two tissue characterisation techniques has been validated to assess plaques behind stents. However, it has been our observation that metallic stent struts appear as dense calcium surrounded by necrotic core, thus, metal stents are artificially included in the calculation of both dense calcium and necrotic core by VH. iMap does not depict necrotic core around the stent, and it shows more clearly the shape of the stent struts classified as calcium (Figure 3).
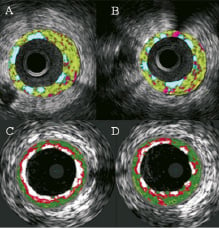
Figure 3. Cross-sections of iMap (top) and VH images (bottom). There is no necrotic core around stent struts by iMap, which shows clearly separated stent struts (A, B). Metallic stent struts appear as confluent dense calcium surrounded by a necrotic core in VH (C, D).
This would be dependent very much on the stent design. In one case example, in same stented segment, in which the guidewire was removed before the IVUS pullbacks for iMap, there was much more dense calcium (28.2 mm3, 27.9 %) shown in VH than by iMap which showed only 5.9 mm3 and 4 %. Furthermore, some metallic stent struts were not detectable by VH compared with greyscale IVUS because they were masked by the artificially imposed grey medial stripe, while in the iMap images, all metallic stent struts with thin fibrous plaque around them were visualised (Figure 4; see online supplementary data).
Imaging of blood
Blood is misclassified by both techniques. It is characterised as a mixture of fibrous and fibrofatty tissue by VH, and depicted as fibrotic tissue in iMap (Figure 5; see online supplementary data).
Conclusions
In this study, in vivo comparison between two different IVUS-based tissue characterisation systems yielded a significant and systematic variability in plaque composition estimates. iMap classified plaque as necrotic tissue in poor signal areas, such as guidewire artefact or acoustic shadowing. VH showed external elastic membrane as a grey medial stripe, while iMap always provided plaque composition results even for very thin plaques. VH tended to overestimate metallic stent struts, and iMap showed thinner stent thickness than VH. Also, there was peri-stent and calcium necrotic core halo in VH that was not seen in iMap. Taken all together, these findings warrant further exploration of these imaging techniques in a large population to find particular areas of clinical usefulness.

