Abstract
Aims: To compare the intravascular ultrasound virtual histology (IVUS-VH) appearance of the polymeric struts of the first (Revision 1.0) and the second (Revision 1.1) generation bioresorbable vascular scaffold (BVS).
Methods and results: IVUS-VH misrepresents polymeric struts as dense calcium (DC) and necrotic core (NC) so that their presence and disappearance could be used as potential artifactual surrogate of bioresorption. DC and NC were assessed in both revisions of the BVS by analysing IVUS-VH from all patients in the ABSORB cohort A (Revision 1.0) and cohort B (Revision 1.1) study who had an IVUS-VH post-treatment and at 6-month follow-up. Post-treatment and 6-month follow-up IVUS-VH results, available in 60 patients (BVS 1.0 n=28; BVS 1.1 n=32), indicated an insignificant rise in DC+NC area compared to baseline with Revision 1.1 (0.10±0.46mm2, p=0.2), whilst a significant reduction was seen with Revision 1.0 (–0.57±1.3 mm2, p=0.02). A significant correlation has been found between the change in the DC+NC area and the change in external elastic membrane area (y=0.68×–0.1; r=0.58, p=0.03).
Conclusions: Based on 6-months IVUS-VH analysis, the BVS 1.1 appears to have a different backscattering signal compared to the BVS 1.0, which may reflect differences in the speed of chemical and structural alteration.
Introduction
The bioresorption process of new bioresorbable vascular scaffolds (BVS) is crucial in the determination of their performance at medium and long-term. A BVS should have enough radial strength to counteract acute vessel recoil following angioplasty and should also be able to maintain its mechanical integrity until late recoil forces subside. The fully resorbable BVS (Abbott Vascular, Santa Clara, CA, USA) has been tested in 30-patient in the first-in-man ABSORB cohort A study and demonstrated excellent long-term clinical results up to two years with a major adverse cardiac event rate of 3.6%.1 However, due to faster degradation, the first generation BVS showed higher late recoil than conventional metallic platform stents.1-3
To prolong the mechanical strength of the scaffold and reduce late recoil, a second generation BVS –Revision 1.1– has recently been introduced, and this is currently undergoing clinical evaluation in the ABSORB cohort B study.4 Compared to the Revision 1.0 which was used in the ABSORB cohort A study, the Revision 1.1 has a smaller maximum circular unsupported surface area,5 a more uniform strut distribution and improved stent retention. Importantly, these changes have not resulted in an increased amount of polymeric material or an increase in strut thickness. Proprietary process changes have been implemented to increase radial strength. In addition, these changes have reduced polymer degradation rates at early time points and thus prolonged mechanical integrity of the scaffold throughout the first few months following implantation.
Intravascular ultrasound virtual histology (IVUS-VH), a tool developed to assess tissue composition of intact native coronary arteries, mis-classifies polymeric stent struts as “dense calcium” and “necrotic core” (white and red in the VH colour code).6 This could potentially be used as surrogate marker of alteration of the polymeric struts and to assess in vivo the degradation process of a BVS.7,8 Shin’s method, for IVUS-VH analysis, allows a more accurate detection of dense calcium and necrotic core so that the bioresorption process can be better explored.9
The aim of this study was; 1) to evaluate temporal changes in the IVUS-VH signal of the BVS 1.1 and BVS 1.0; 2) to assess the correlation between the changes in IVUS-VH signal for BVS 1.1 and 1.0, and the change in external elastic membrane (EEM) area.
Methods
Study design
All patients from cohort A and cohort B of the ABSORB trial with paired post-BVS implantation and 6-month follow-up IVUS-VH data were included. The ABSORB cohort A study is described elsewhere.1 In brief, for cohort A and cohort B, patients were suitable for inclusion if they were older than 18 years, with adiagnosis of stable, unstable or silent ischaemia. All treated lesions were de novo lesions in a native coronary artery with a reference vessel diameter of 3.0 mm, with a percent diameter stenosis ≥50% and <100% and a thrombolysis in myocardial infarction (TIMI) flow grade of ≥1. The BVS 1.0 was used in patients in cohort A, whilst Revision 1.1 was used in cohort B.4 Major exclusion criteria were: patients presenting with an acute myocardial infarction, unstable arrhythmias or patients who had left ventricular ejection fraction ≤30%, restenotic lesions, lesions located in the left main coronary artery, lesions involving a side branch >2 mm in diameter, and the presence of thrombus or another clinically significant stenosis in the target vessel. Both ABSORB trials were approved by ethics committee at each participating institution and each patient gave written informed consent before inclusion.
Study device
The BVS has an amorphous poly-DL-lactide (PDLLA) coating that contains and controls the release of the anti-proliferative drug everolimus. The scaffold body is made of semi-crystalline poly-L-lactide (PLLA). PLA is completely degraded via hydrolysis and bioresorbed via the lactate shuttle. There are no differences in polymeric material, drug dose, drug release or strut thickness between BVS Revisions 1.0 and 1.1. Of note, the BVS Revision 1.1 has a smaller maximum circular unsupported surface area compared to Revision 1.0.5 Processing changes in Revision 1.1 have resulted in higher and prolonged mechanical strength and stability post implantation. (Figure 1)
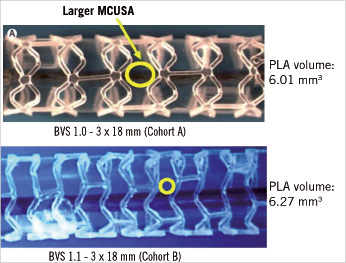
Figure 1. Although the scaffold design is different between revision 1.0 and revision 1.1, the content of polymer is nearly the same. MCUSA: maximum circular unsupported surface area; BVS: bioresorbable vascular scaffold; PLA: polylactide
BVS implantation procedure
In both cohorts, lesions were treated with routine interventional techniques that included mandatory pre-dilatation using a balloon shorter than the study device and 0.5 mm less in diameter. All patients were pre-treated with aspirin and a loading dose of at least 300 mg of clopidogrel was administered according to local hospital practise. After the procedure, all patients received aspirin ≥75 mg for the study duration (five years) and clopidogrel 75 mg daily for a minimum of six months. Anticoagulation and glycoprotein IIb/IIIa use was according to local hospital practice.
Imaging procedure and acquisition
IVUS-VH post-BVS implantation and at 6-month follow-up were obtained from patients from both cohorts. Imaging techniques were acquired simultaneously with a phased array 20MHz intravascular ultrasound catheter (EagleEye™; Volcano Corporation, Rancho Cordova, CA, USA) using an automated pullback of 0.5 mm per second. Four tissue components (necrotic core – red; dense calcium – white; fibrous – green; and fibrofatty – light green) were identified with autoregressive classification systems.10,11 Each individual tissue component was quantified and colour coded in all IVUS cross sections as previously described.10 All IVUS-VH analyses were performed offline using pcVH 2.1 (Volcano Corporation, Rancho Cordova, CA, USA). We carefully matched the region received BVS implantation using anatomical markers to compare the post implantation and 6-month follow-up IVUS-VH images. Regions received non-BVS stent implantation were not analysed. For each cross section, polymeric struts were detected as areas of apparent “dense calcium” and “necrotic core”. The quantitative changes in dense calcium (DC) and necrotic core (NC) content were used as a surrogate marker of alteration of the polymeric struts, as previously described.1,7,12,13
Only changes in DC and NC, which can be seen as the fingerprint of the BVS, were required, therefore the lumen contour was drawn around the IVUS catheter without following the leading edge of the interface lumen intima, as previously described by Shin et al.9 Using this approach, the BVS struts closest to the lumen were better detected and recognised as DC and NC by the VH software and the ones overlying small plaques (thinner side of an eccentric plaque) were not obscured by the imposed grey medial stripe seen with IVUS-VH. (Figure 2) We also recorded in all patients the interface between plaque+media and the adventitia (EEM area). Lumen area and plaque area cannot be recorded using this method.
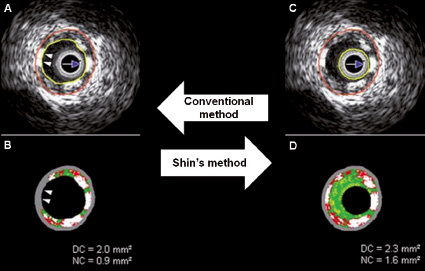
Figure 2. The conventional method (A and B) does not show some necrotic core and dense calcium (two white arrow heads), hidden by the grey medial stripe (two white arrow heads), that are showed by the Shin’s method (C and D). DC: dense calcium, NC: necrotic core
Statistical analysis
Discrete variables are presented as counts and percentages. Continuous variables are presented as mean ±standard deviation (SD). The DC and NC values have been statistically tested separately post-procedure and 6-month follow-up. The VH fingerprint of the BVS in the artery was defined as the sum of the changes in DC and NC, as IVUS-VH detects stent struts as dense calcium surrounded by necrotic core halo.6 The calculation of the changes between post-treatment and six months was as follows: mean six month area minus mean post-procedure area for both NC and DC. Paired comparisons between post-procedure and 6-month follow-up were performed using the Wilcoxon signed rank test. Comparisons between groups were assessed using non parametric tests, whilst correlations between parameters were performed by using a Spearman test. A two-side p-value of less than 0.05 indicated statistical significance. Statistical analyses were performed with use of SPSS 13.0 software (SPSS Inc., Chicago IL, USA).
Results
The comparison between post-procedure and 6-month follow-up IVUS-VH included 28 patients from cohort A and 32 patients from cohort B. (Figure 3)
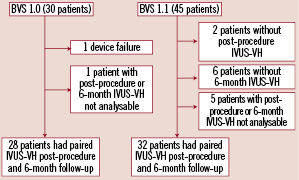
Figure 3. Flow chart of paired IVUS-VH data available for both cohorts.
Table 1 shows clinical and angiographic data in both cohorts.
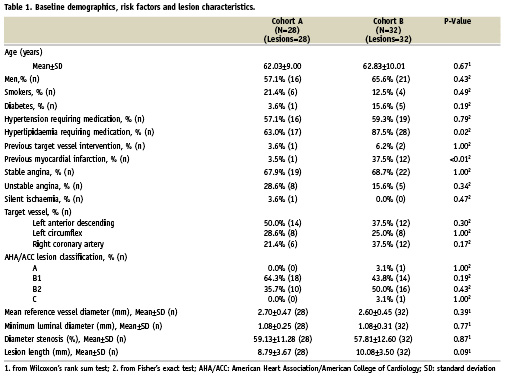
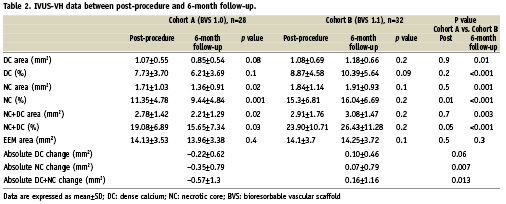
Compositional changes from post-treatment to six month follow-up by IVUS-VH (Table 2).
In cohort A, there was a relative 20% decrease in both DC and NC (p=0.08 and p=0.02) between post-procedure and 6-month follow-up. Conversely, over the same time period in cohort B, a small relative increase in DC and NC of 9% (p=0.2) and of 4% (p=0.1) respectively, was observed. (Figure 4) At 6-month follow-up, while in cohort A there was regression in the calcified pattern in 16 out of 27 patients (59%) and in the necrotic pattern in 20 out of 27 patients (74%), in cohort B there was regression in the calcified pattern in 11 out of 26 patients (42%) and in necrotic pattern in seven out of 26 patients (27%). Overall, the absolute DC+NC decrease was significantly larger in cohort A than cohort B, suggesting an earlier IVUS-VH alteration of the polymeric struts with the BVS 1.0 as compared to the BVS 1.1.
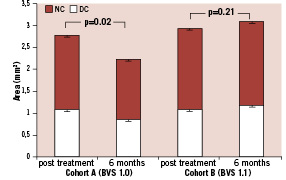
Figure 4. DC and NC area at six months compared to post-treatment in BVS 1.0 and BVS 1.1. Error bars portray standard error of the mean. DC: dense calcium; NC: necrotic core
Grey-scale intravascular ultrasound data and correlation with IVUS-VH alteration of BVS 1.0 and 1.1
On average, at six month follow-up, the EEM area did not significantly differ from post-treatment status in both cohorts (Table 2).
Classifying patients according to reduction in the EEM area at 6-month follow-up, we analysed changes in absolute area of DC and NC core in both cohorts. In patients from cohort A, those with reduction of the EEM area at 6-month (n=15) showed a significantly lower absolute area change of NC compared to other patients (n=13) (-0.69±0.73 mm2 vs. 0.05±0.68 mm2, p=0.017) (Figure 5). Reduction of DC was no different (-0.40±0.63 mm2 vs. –0.02±0.56 mm2, p=0.1). Overall no correlation was found between changes in EEM area and the sum of absolute change in DC+NC area (Figure 6A).
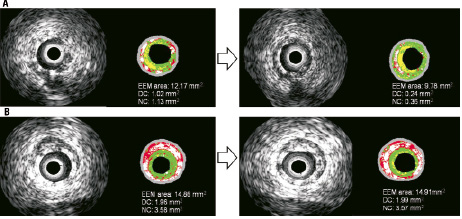
Figure 5. In the A panels, an extreme example of bioresorption at 6-months correlated with EEM area reduction. In the B panels, the persistence of the stent at six months is not associated with EEM area reduction. With the Shin method, blood surrounding ultrasound catheter is detected as fibrous and fibrous-fatty tissues. The boundaries of the lumen area are not depicted or superimposed on the figures. EEM: external elastic membrane; DC: dense calcium; NC: necrotic core
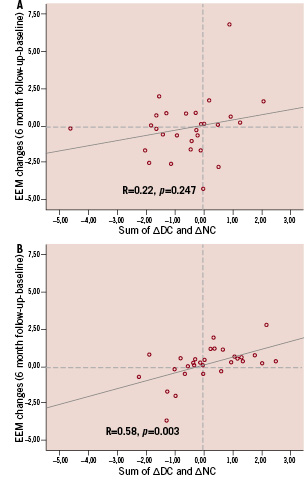
Figure 6. Bioresorption of the scaffold, measured by sum of absolute change in dense calcium and necrotic core area is significantly correlated with change of EEM area in cohort B (Panel B), but not in cohort A (Panel A). In the lower left quadrant of both panels, patients with bioresorption of BVS and reduction of EEM area are shown. EEM: external elastic membrane; DC: dense calcium, NC: necrotic core; BVS: bioresorbable vascular scaffold
On the other hand, in patients from cohort B with a reduction in the EEM area (n=16), there was a significantly lower change in absolute area of DC and NC at follow-up compared to other patients (n=16) (respectively for DC –0.12±0.41 mm2 vs. 0.30±0.42 mm2, p=0.007; for NC –0.38±0.84 mm2 vs. 0.50±0.45 mm2, p<0.001). In the overall population, the change in DC+NC area correlated significantly with the change in EEM area. (R=0.58, p=0.003). (Figure 6B).
Discussion
The major findings of our study are: 1) the changes in design, degradation rate and mechanical durability of the BVS 1.1 compared to BVS 1.0 significantly influenced its IVUS-VH changes over a period of six months (i.e., less reduction in DC and NC at six months); 2) a relationship exists between change overtime in EEM area and in DC+NC area.
Kim et al have previously shown that metallic stents eluting sirolimus and paclitaxel introduce artifacts in IVUS-VH images, that interfere with the classification of plaque behind the struts.6 Normally struts of drug-eluting stents appear as DC, surrounded by a red halo. Although the BVS is made of non-metallic materials, it was also recognised by IVUS-VH software as DC and NC. For this scaffold, the presence of “pseudo” DC and NC could be used as surrogate marker of alteration of the polymeric struts.1,7,8,12-14 Garcia-Garcia et al have already shown in a sub-study of ABSORB cohort A trial that polymeric struts are identified with radiofrequency backscattering as calcific structures. Using IVUS-VH, it has been shown that there was an important increase in DC and NC immediately after BVS implantation.7 This sudden change in DC and NC has been attributed to the introduction of polymeric struts and might represent their VH fingerprint. Our data confirm that polymeric struts appear as DC and NC and that IVUS-VH is a potential approach to semi-quantify or state the presence of the polymer.
The ability of IVUS-VH to recognise polymeric struts is important also to potentially follow the mechanical support or bioresorption process. Our study demonstrated that while for the BVS Revision 1.0 there is a reduction in DC and NC after six months,7 for the BVS Revision 1.1 the IVUS-VH signature appears to be unchanged over the same period. This stable backscattering signal with the BVS 1.1 suggests unchanging polymeric structure and/or stable mechanical properties due to slower degradation in vivo, as intended. The first revision of the BVS showed slightly higher acute recoil than conventional metallic platform stents2 and at six months an 11.7% reduction in scaffold area of the BVS 1.0 area was documented.3 Of note, the longer-lasting mechanical integrity of the BVS Revision 1.1, –as suggested by unaltered IVUS-VH signature over a period of six months, may prevent this loss in structural integrity and reduction in scaffold area.1,15 This observation of constancy in radiofrequency backscattering of BVS 1.1 is largely confirmed by the absence of qualitative alterations of the appearance of the polymeric struts of BVS 1.1 with optical coherence tomography (OCT).4 They uniformly keep their “preserved box” appearance, whereas with the BVS 1.0 only 3% of preserved box were present at six months follow-up.12
Our data also showed that the change in DC+NC area detected by IVUS-VH may be associated with shrinkage of the EEM area. For Revision 1.1, we found a significant correlation between the reduction of the EEM area at follow-up and changes in the sum of DC and NC. In particular, it seems to be a trend between the EEM shrinkage and the positive/negative value of sum of changes in DC and NC. In the case of Revision 1.0, the absence of any relationship between the change in IVUS-VH and the change in EEM area may be the result of a different phenomenon, or it may relate to a broader biological and/or sample variability; in particular looking at Figure 6, the two lines for both the cohorts seem similar and one is significant but not the other probably because of less variability. It is interesting to note that for both scaffolds the IVUS-VH changes (and potentially the bioresorption process) are not uniform in all patients. It depends not only on the hydrolysis of the scaffold, but probably also on the nature of the underlying plaque (composition and inflammatory state), and the further recruitment of macrophages to the site of the scaffold implantation. OCT of the presence of macrophages surrounding the scaffold, and the analysis of plaque composition behind the scaffold will enhance the understanding of the bioresorption process of the BVS.
In conclusion, IVUS-VH is a clinical method that can detect differences in behaviour between BVS Revision 1.0 and 1.1. Their IVUS-VH fingerprints may be influenced by changes in mechanical design or in product processing. Stability in the IVUS-VH signal of the BVS 1.1 through to 6-months follow-up might suggest a more durable mechanical integrity than the BVS 1.0.
Limitations
We acknowledge that the classification tree of IVUS radiofrequency analysis has not been validated for polymeric struts. We did not collect data about change in fibrous tissue, because it is known that polymeric struts only appear as dense calcium and necrotic core on IVUS-VH. At the same time, using Shin’s method, we did not collect data on changes to the lumen area and plaque burden. Changes in DC and NC at follow-up are probably not only related to the BVS, but also to the natural history of the atherosclerotic disease.

