
Abstract
Aims: We aimed to compare intravascular ultrasound with virtual histology (VH-IVUS), optical coherence tomography (OCT) and near-infrared spectroscopy (NIRS) for their ability to quantify the true amount and characterise the nature of released plaque material during bioresorbable vascular scaffold (BVS) implantation into right coronary artery (RCA) lesions using a distal occlusion and aspiration device.
Methods and results: Seventeen patients underwent BVS implantation into the right coronary artery under distal protection with intracoronary imaging using VH-IVUS, OCT and NIRS. The amount of released plaque material and its lipid content (LC) were determined. Necrotic core volume and minimal fibrous cap thickness correlated with the amount of released plaque material (r=0.80 and r=–0.65, respectively) and its LC (r=0.75 and r=–0.78, respectively), but not maximal lipid core burden index (LCBI). OCT-identified thin-cap fibroatheromata (TCFA) were associated with the greatest amount of released plaque material compared to non-TCFA (46.8 [29.0;49.2] mg vs. 14.2 [11.3;19.4] mg; p=0.003) and LC (4.4 [4.0;4.8] mg vs. 2.0 [1.8;2.5] mg; p=0.000).
Conclusions: VH-IVUS and OCT but not NIRS parameters quantify and characterise the amount of released plaque material. TCFA is associated with the highest amount of released plaque material and may therefore benefit from the use of protection devices.
Abbreviations
a.u.: arbitrary unit
BVS: bioresorbable vascular scaffold
CAD: coronary artery disease
LC: lipid content
LCBI: lipid core burden index
LCBIlesion: lipid core burden index of the lesion
maxLCBI4mm: maximal lipid core burden index over any 4 mm segment within the lesion
MI: myocardial infarction
NIRS: near-infrared spectroscopy
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
RCA: right coronary artery
TCFA: thin-cap fibroatheroma
VH-IVUS: intravascular ultrasound with virtual histology
Introduction
Lumen gain by percutaneous coronary intervention (PCI) is achieved by stretching of the vessel with the culprit lesion, dissection, plaque redistribution and embolisation of plaque debris. Microembolisation can induce periprocedural myocardial infarction (MI) in 2-50% of cases1. The incidence of periprocedural MI varies depending on the culprit lesion, the type of intervention and the biomarkers/parameters used for its diagnosis2. The clinical consequences of periprocedural microembolisation of plaque debris and soluble thrombogenic, vasomotor and inflammatory substances, which contribute to microvascular obstruction3-5, range from asymptomatic increases in cardiac biomarkers to severe, life-threatening situations. The prognostic relevance of periprocedural MI is still controversial6; however, there is consensus to limit myocardial injury as much as possible.
Intracoronary imaging modalities provide insight into the vascular wall and identify certain plaque phenotypes which are particularly susceptible to distal embolisation7: intravascular ultrasound with virtual histology (VH-IVUS) can quantify the necrotic core8,9, optical coherence tomography (OCT) can detect lesions with a thin fibrous cap (thin-cap fibroatheroma [TCFA])10, and near-infrared spectroscopy (NIRS) quantifies the lesion’s maximal lipid core burden11-13. These imaging modalities and their distinct parameters have been compared to each other14-16 and correlated with post-interventional increases of cardiac biomarkers8,10,17,18. So far, these imaging parameters have neither been correlated with the true amount of in vivo released plaque material nor analysed for their potential to characterise the nature of the embolised material. An imaging parameter, which accurately quantifies the amount and characterises the nature of released plaque material, might allow better prediction of clinical events.
The efficacy of embolisation protection devices during PCI continues to be the subject of controversy, especially in native vessel PCI19, although their use in saphenous vein grafts is supported by strong evidence and recommended in current guidelines. Apart from a potentially beneficial effect, embolic protection devices offer the opportunity to capture iatrogenically released plaque material, which otherwise embolises into the downstream coronary microcirculation3; obviously, with the capture of the released plaque material, the lesion phenotype can no longer be correlated to biomarker release or clinical endpoints. Implantation of bioresorbable vascular scaffolds (BVS) in particular has been associated with a higher incidence of periprocedural MI by increased periprocedural plaque embolisation or side branch occlusion due to a higher strut width and thickness, compared to metal stents20. Here, we aimed to compare VH-IVUS, OCT and NIRS for their ability to quantify the true amount and characterise the nature of released plaque material during BVS implantation into right coronary artery (RCA) lesions using a distal occlusion and aspiration device.
Methods
The study was approved by the institutional review board (No. 07-3387) and conducted in accordance with the ethical guidelines of the Declaration of Helsinki 1975. Patients gave written informed consent prior to their inclusion in the study. Twenty patients with stable CAD underwent BVS implantation (Absorb™; Abbott Vascular, Santa Clara, CA, USA) without predilatation into the RCA using an embolic protection device (GuardWire®; Medtronic, Minneapolis, MN, USA). Only patients with RCA lesions were enrolled for anatomical reasons, allowing the use of a distal balloon occlusion and aspiration protection device in order to obtain coronary aspirate with its particulate and soluble plaque material. Troponin I increase within 48 hrs was measured in order to verify protection against periprocedural embolisation. Inflammatory parameters, i.e., counts of leukocytes, C-reactive protein, procalcitonin and interleukin 6, were measured before BVS implantation and within 48 hrs. Comparison of baseline values as well as the maximal increase after the procedure was made between TCFA and non-TCFA.
INTRACORONARY IMAGING
VH-IVUS (Volcano Eagle Eye® Platinum RX Digital IVUS Catheter; Volcano Corporation, San Diego, CA, USA), OCT (C7 Dragonfly™ Imaging Catheter; St. Jude Medical, St. Paul, MN, MA, USA) and NIRS (True Vessel Characterization Imaging System™; Infraredx, Burlington, MA, USA) were performed before and after BVS implantation using standard techniques (Figure 1A-Figure 1L)8,14,17.
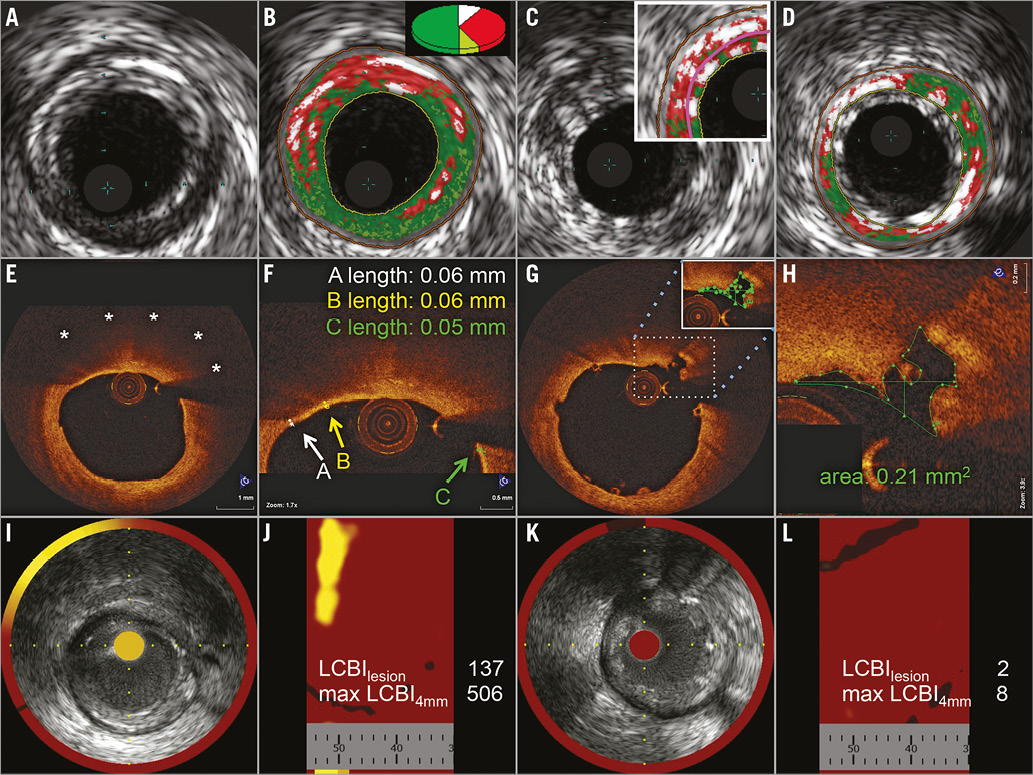
Figure 1. Pre- and post-interventional VH-IVUS, OCT and NIRS. Corresponding pre-interventional (left two columns) images of VH-IVUS (A-D), OCT (E-H) and NIRS (I-L), showing a TCFA with a large necrotic core (B) in VH-IVUS (dark green: fibrotic, light green: fibro-fatty, red: necrotic core, white: dense calcium), a lipid pool of 180° (E, stars) covered by a thin fibrous cap in OCT (F, arrows) and a high maxLCBI4mm in NIRS (I & J). Post-interventional (right two columns) images show artefacts by BVS struts, misinterpreted as dense calcium by IVUS (C). The plaque volume was determined behind the struts (D), according to Brugaletta et al30. OCT shows well-apposed BVS struts and a plaque rupture (G), the circumference of which was manually drawn in every slice (H) over the total length of the treated lesion to allow calculation of the amount of intimal injuries; NIRS shows a reduction of LCBI (K & L).
Pre-interventional imaging parameters, which have been proposed to predict periprocedural MI, were pre-specified to be correlated with the amount of released plaque material and its lipid content (LC), i.e., necrotic core volume in VH-IVUS9 (Figure 1A, Figure 1B), minimal fibrous cap thickness in OCT17 (Figure 1E, Figure 1F), and maximal lipid core burden index over any 4 mm segment within the lesion (maxLCBI4mm) in NIRS11,12 (Figure 1I, Figure 1J). In addition, a dichotomous comparison was performed between OCT-identified TCFA, defined as lesions with a lipid arc >180° and a fibrous cap thickness <65 µm21, and non-TCFA.
Also, post-interventional imaging parameters were correlated with the amount of released plaque material and its LC. ∆ Plaque volume was calculated as the difference between pre- and post-interventional plaque volume in VH-IVUS (Figure 1B-Figure 1D). In OCT, new endothelial injuries were quantified by volumetry (Figure 1G, Figure 1H). Therefore, in every slice of the BVS-treated lesion, plaque ruptures and dissections were identified, and the area was measured by manually drawing the circumference of each injury. The sum of all measured areas was multiplied by the slice thickness of 0.2 mm, which resulted in a calculated volume. This parameter was termed “volume of intimal injury”. In NIRS (Figure 1I-Figure 1L), the difference between the pre- and post-interventional lipid core burden index of the lesion (∆ LCBIlesion) was calculated.
QUANTIFICATION OF RELEASED PLAQUE MATERIAL AND ITS LIPID CONTENT
Coronary aspirate blood was obtained during distal balloon occlusion through an aspiration catheter and directly filtered through a 40 μm mesh filter to separate particulate plaque material. Particulate plaque material was rinsed with NaCl solution (0.9%) in order to clear it from remaining erythrocytes, which would have disturbed our further analysis, i.e., the determination of total lipid content, as erythrocytes contain a high proportion of cholesterols. Released particulate plaque material was weighed and characterised by Pappenheim staining of a smear of residual plaque material, attached to the plastic material of a syringe22. Simultaneously to coronary aspiration, venous blood samples were obtained.
LC was determined in particulate plaque material, in coronary aspirate plasma and in venous plasma by lipid extraction, as described by Bligh and Dyer23. The amount of released plaque material and its LC were defined as follows: amount of released plaque material=weightparticulate plaque material+(LCcoronary aspirate plasma-LCvenous plasma), LC=LCparticulate plaque material+(LCcoronary aspirate plasma-LCvenous plasma).
Statistical analysis
Image analyses were performed by two independent investigators blinded to the results of the ex vivo plaque analyses. The imaging parameters of both investigators were averaged; inter-observer agreement was good (weighted kappa >0.61; data not shown). Since non-normality was shown for some parameters (Shapiro-Wilk test, data not shown), all continuous variables are presented as median (lower quartile; upper quartile) and categorical variables as frequencies (percentages). Spearman’s correlations were calculated. Comparison between two groups was performed using the Mann-Whitney U test. Due to the small sample size, exact permutation tests were performed to calculate p-values. All statistics were performed with SPSS software (SPSS Statistics, Version 19.0; IBM Corp., Armonk, NY, USA). A p-value <0.05 was considered significant.
Results
DEMOGRAPHICS AND PROCEDURAL DATA
Patient characteristics and procedural data are presented in Table 1. All procedures were performed uneventfully. Asymptomatic peri-interventional troponin I increase to threefold or more above the upper limit of normal within 48 hrs occurred in three cases. Two of these cases had a small, adherent white thrombus in post-interventional OCT, explaining a modest increase of troponin despite the use of a protection device; however, all three cases were excluded from further analysis. There was no significant difference between baseline and maximal increase of inflammatory parameters between TCFA and non-TCFA (data not shown).
In four patients, two BVS were implanted either by one single (n=3) or two separate distal occlusions (n=1). In this case, captured plaque material of both separate occlusions was collected in the same cryotube for further analysis. In one single case, post-interventional OCT revealed partial strut malapposition that required focal post-dilatation, which was not performed under distal occlusion. However, there was no increase in post-interventional troponin in this case. In all cases, post-interventional angiography revealed normal coronary blood flow, filling the distal coronary bed completely; there were no side branch occlusions.
QUANTIFICATION OF THE AMOUNT OF RELEASED PLAQUE MATERIAL AND ITS LIPID CONTENT
The amount of released plaque material was 26.4 (13.1;39.5) mg and its LC was 2.9 (1.9;4.3) mg. Histologic analysis revealed clusters of foam cells surrounded by platelets in all cases (Figure 2).
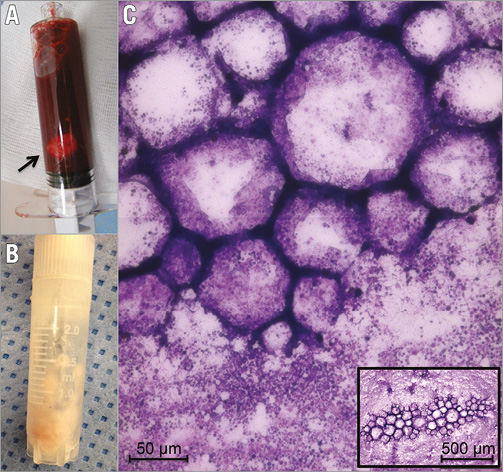
Figure 2. Released plaque material. Capture of released plaque material, showing a syringe filled with coronary blood and particulate plaque material (A, arrow), a cryotube with separated particulate plaque material, cleared from erythrocytes (B), and a Pappenheim-stained smear, notably clusters of foam cells surrounded by aggregated platelets (C).
RELATION BETWEEN IMAGING PARAMETERS AND THE AMOUNT OF RELEASED PLAQUE MATERIAL AND ITS LIPID CONTENT
In VH-IVUS, all lesions consisted of at least three consecutive slides with a necrotic core >10%, OCT identified seven lesions as TCFA and NIRS detected seven lesions with a maxLCBI4mm >500. Interestingly, these lesions did not correspond to each other. Pre- and post-interventional imaging parameters and correlations of all analysed imaging parameters are presented in Table 2 and Table 3. In the following paragraphs we focus on the pre-specified imaging parameters.
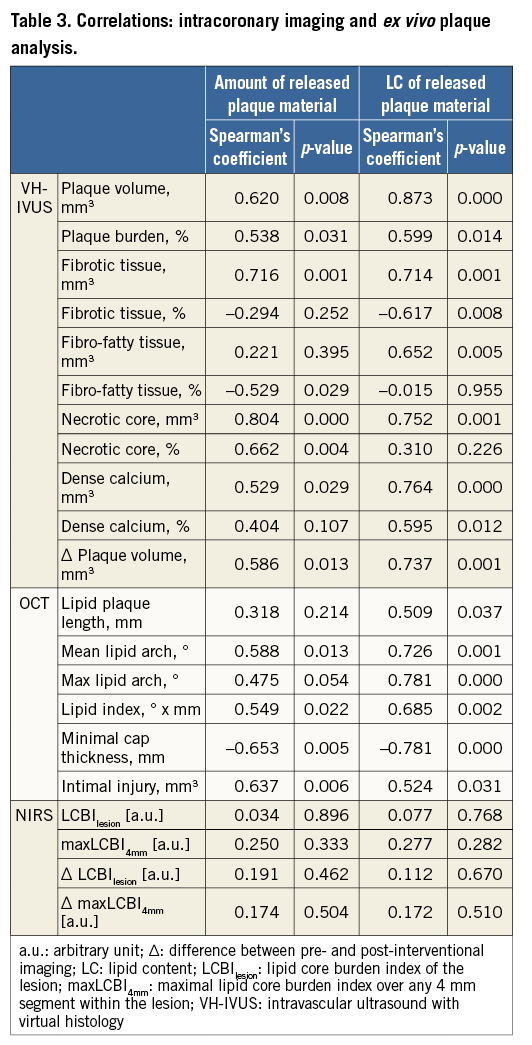
Pre-interventional necrotic core volume correlated with the amount of released plaque material and with its LC (Figure 3A). Minimal fibrous cap thickness correlated inversely with the amount of released plaque material and with its LC (Figure 3B). In contrast, maxLCBI4mm correlated neither with the amount of released plaque material nor with its LC (Figure 3C).
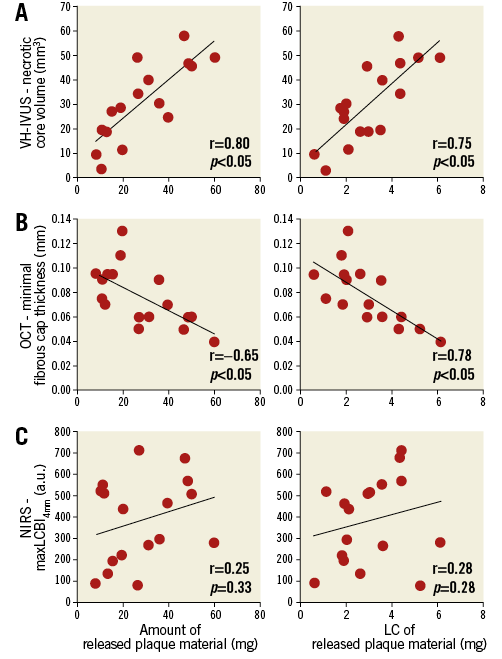
Figure 3. Quantification and characterisation of released plaque material. Correlations between the amount of released plaque material (left column) and its lipid content (right column) with pre-interventional imaging parameters of VH-IVUS (A), OCT (B) and NIRS (C).
The comparison of OCT-identified TCFA versus non-TCFA (Figure 4) revealed a higher amount of released plaque material (46.8 [29.0;49.2] vs. 14.2 [11.3;19.4] mg, p=0.003) and a greater LC (4.4 [4.0;4.8] vs. 2.0 [1.8;2.5] mg, p=0.000) in TCFA than in non-TCFA as well as a greater necrotic core volume (46.8 [42.8;49.2] vs. 19.1 [13.3;26.5] mm3, p=0.000), while there was no significant difference in maxLCBI4mm (506 [276;624] vs. 367 [201;501] a.u., p=0.364).
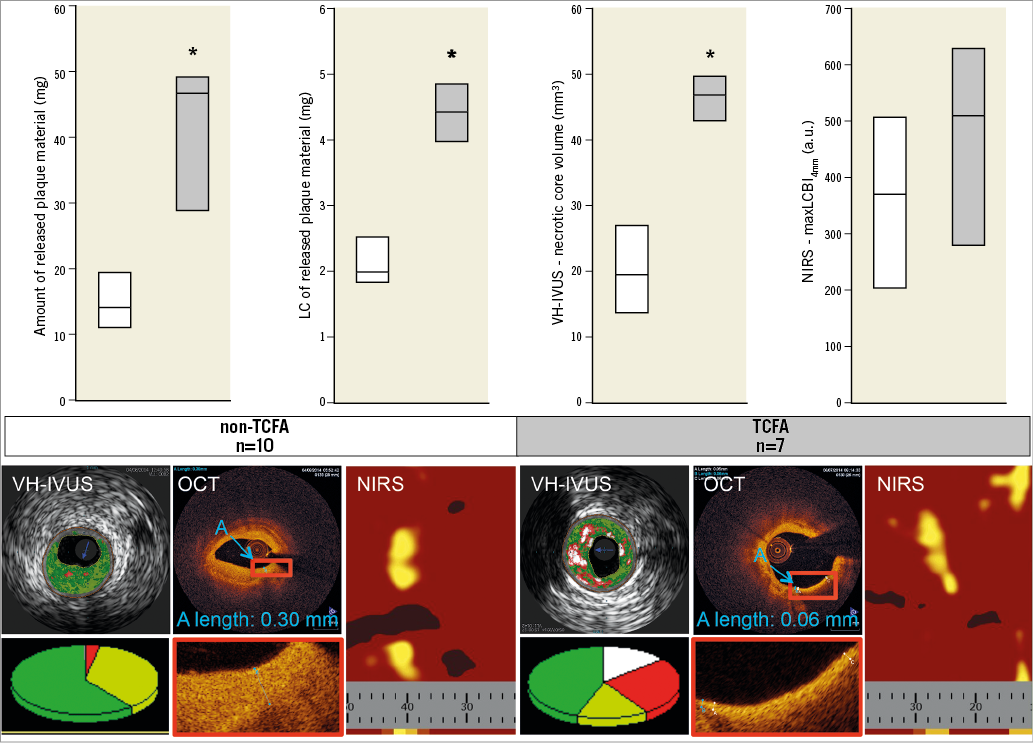
Figure 4. Comparison between non-TCFA and TCFA. TCFA (grey) are associated with a greater amount and a higher LC of released plaque material than non-TCFA (white). As shown by diagrams and exemplary images of all modalities, TCFA have higher necrotic core volumes than non-TCFA, while there is no significant difference in maxLCBI4mm. VH-IVUS - dark green: fibrotic, light green: fibro-fatty, red: necrotic core, white: dense calcium; data are expressed as median (upper quartile; lower quartile). * p<0.05 vs. non-TCFA.
Post-interventional VH-IVUS-derived ∆ plaque volume correlated with the amount of released plaque material and with its LC. Likewise, the OCT-derived volume of intimal injuries correlated with the amount of released plaque material and with its LC. In contrast, ∆ LCBIlesion correlated neither with the amount of released plaque material nor with its LC (Table 3).
Discussion
Our study compared current intracoronary imaging modalities for their potential to quantify the amount and characterise the nature of in vivo released plaque material during BVS implantation in patients with stable CAD by ex vivo analysis of captured plaque material. The amount of released plaque material is reflected by necrotic core volume in VH-IVUS and by minimal fibrous cap thickness in OCT, but not with NIRS-derived maxLCBI4mm.
QUANTIFICATION OF THE AMOUNT OF RELEASED PLAQUE MATERIAL
Clinical studies suggest necrotic core in VH-IVUS, and minimal fibrous cap thickness and TCFA in OCT as predictors for periprocedural MI by correlation with cardiac biomarkers or clinical events9,10,17. The present study extends such studies to estimate periprocedural embolisation by correlation of distinct imaging parameters with the true amount of in vivo released plaque material. The OCT-identified TCFA, which is characterised by a high necrotic core volume in VH-IVUS, is associated with a greater amount of released plaque material than a non-TCFA.
In contrast to VH-IVUS and OCT, NIRS only shows a tendency to correlate with the amount of released plaque material, although the predominant cells observed in the Pappenheim-stained smear of released plaque material are lipid-rich foam cells, and NIRS has been reported to detect lipids specifically. In line with our observations, the recent large-scale randomised multicentre CANARY trial also did not confirm NIRS parameters as predictors for periprocedural MI or a reduction of periprocedural MI by use of protection devices in lesions presenting with a maxLCBI4mm >600 a.u.18. Both the missing correlation to the true amount of released plaque material in our study and the results of the CANARY trial may be explained by the inability of NIRS to determine the depth of a lipid pool14.
CHARACTERISATION OF THE NATURE OF RELEASED PLAQUE MATERIAL
Until now, imaging parameters have been verified by comparison to pathology specimens24-27 or indirectly by comparison of different modalities to each other regarding their potential for lipid detection14-17. Necrotic core volume (VH-IVUS) is associated with clefts filled with cholesterol and foam cells28. TCFA are histologically characterised as lipid-rich plaques covered by only a thin fibrous cap29, which can be measured by OCT26. We here present an association of both VH-IVUS and OCT parameters with the LC of in vivo released plaque material.
In addition to the inability of NIRS to quantify the amount of released plaque material, NIRS parameters do not even correlate with its LC. This may be explained by the already mentioned inability to offer a proper axial resolution and a total lipid volume.
POST-INTERVENTIONAL QUANTIFICATION OF THE AMOUNT OF RELEASED PLAQUE MATERIAL AND ITS LC
The difference between pre- and post-interventional imaging parameters is expected to reflect the amount of released plaque material, although this has not been thoroughly validated. In line with this assumption, VH-IVUS-derived ∆ plaque volume correlates with the amount of released plaque material and with its LC. Likewise, the OCT-derived volume of intimal injuries correlates with the amount of released plaque material and its LC. Consequently, VH-IVUS and OCT may allow a post hoc quantification of released plaque material. Nevertheless, our conclusion is based on correlations between calculated imaging parameters and the amount of released plaque and its LC and is, therefore, only hypothesis-generating.
Since pre-interventional NIRS parameters reflected neither the amount nor the nature of released plaque material, we could not expect and indeed did not determine a correlation with post-interventional ∆ LCBIlesion. A post-interventional plaque shift and rearrangement of lipid cores from deeper to superficial areas might cause such a lack of correlation. Nevertheless, NIRS revealed an overall reduction of LCBIlesion after BVS implantation. This fact confirms prior studies which reported a PCI-related reduction of LCBI13,18. The reduction of LCBI per se supports the idea of periprocedural release of lipids, whereas here we demonstrate that this reduction reflects neither the quantity nor the quality of in vivo released plaque material.
Limitations
Our study was limited to a small number of patients with stable CAD and RCA lesions; patients with PCI of saphenous vein grafts or with acute MI, who have an even higher risk for periprocedural embolisation, were excluded. The complexity of the procedure with three different imaging catheters performed prior to and after BVS implantation under distal occlusion/protection might have induced a patient selection bias. Clinical endpoints for periprocedural MI, such as increases in troponin I or adverse clinical outcomes, are lacking due to the study’s observational nature without randomisation for the use of a protection device.
The selection of patients presenting with stable CAD may be the most important reason to explain why NIRS was incapable of differentiating plaques with a high risk of embolisation from those without. This fact is in contrast to most published literature. Nevertheless, most studies were performed in patients with acute coronary syndromes who, per se, have a higher risk of embolisation. The fact that the CANARY trial enrolled patients with stable angina, silent ischaemia and stabilised acute coronary syndromes might have been an additional factor, explaining the failure of NIRS to predict periprocedural MI in this large-scale, randomised study18. Although we observed a post-interventional reduction of LCBI, we could not demonstrate a correlation with the quantity of in vivo released plaque material. Of note, artefacts of the BVS struts might have hampered NIRS analysis, which has not been validated so far for lipid detection behind polymeric BVS struts.
Apart from and in addition to the pre-specified necrotic core volume, other VH-IVUS parameters correlated with the amount of released plaque material and its LC (Table 3). This might be explained by the low predictive value of VH-IVUS to distinguish different plaque components.
The OCT-derived quantification of intimal injuries is a new method and must be validated in future studies. Nevertheless, the current results by two independent, blinded investigators were conclusive.
Conclusion
VH-IVUS and OCT but not NIRS parameters can quantify and characterise the amount of in vivo released plaque material and its LC in the setting of BVS implantation into RCA lesions for stable CAD. TCFA is associated with the highest amount of plaque material released during BVS implantation, thereby potentially benefiting from the use of protection devices during intervention.
| Impact on daily practice VH-IVUS and OCT but not NIRS parameters can quantify and characterise the amount of released plaque material and its LC, allowing a lesion-specific risk assessment, and thus potentially leading to different treatment algorithms, such as the selective use of protection devices, plaque stabilising medication and/or additional antithrombotic treatment. TCFA appear most vulnerable with the highest amount of plaque material released during BVS implantation, thereby potentially benefiting from the use of protection devices. |
Funding
H. Hildebrandt was supported by a research grant from the Medical Faculty of the University Duisburg-Essen (IFORES 10+2).
Conflict of interest statement
The authors have no conflicts of interest to declare.

