Abstract
Background: Little is known about the correlation between modifications in plaque composition at stent edges and the changes in vessel geometry. This study sought to evaluate, by serial greyscale intravascular ultrasound (IVUS) and Virtual Histology® intravascular ultrasound (VH®-IVUS), the modifications in plaque composition at the edges of drug-eluting and bare metal stents and the correlation of these findings with changes in the measurements of vessel, lumen and plaque area at those segments.
Methods and results: Single-centre, prospective and randomised (1:1) evaluation of 40 patients with acute coronary syndrome treated with bare metal (Driver™; Medtronic, Santa Clara, CA, USA; n=20 patients) or drug-eluting stents (Cypher™; Cordis, Miami Lakes, FL, USA; n=20 patients). IVUS and VH-IVUS assessments were done post-procedure and at nine months. Primary endpoint included the modification in vessel, lumen and plaque area and in the composition of the plaque in the mean time between the baseline and follow-up procedure. At the proximal edge of the vessel treated with the Cypher™ stent, a trend toward positive vessel remodelling (∆=+0.6 mm2, p=0.06) was observed while at the distal edge, less plaque growth (∆=+0.2 mm2 vs. ∆=+1.1 mm2, p<0.001), resulted in a larger lumen area at follow-up. By VH, there was a marked reduction in the percentage of fibrotic tissue and necrotic core at the edges of both stents and a positive correlation was seen between increase in percentage of fibro-fatty component and increase in plaque area (r=0.78, p=0.01).
Conclusion: Patients treated with drug-eluting stents (DES) experienced less plaque growth, especially at the distal edge of the stents. Modifications in plaque composition, with increase in fibrofatty tissue component, may partially explain these findings.
Introduction
The advent of drug-eluting stents (DES) represented a major breakthrough in interventional cardiology in the past decade. This novel technology significantly reduced in-stent neointimal tissue formation and consequently minimised the occurrence of restenosis as compared to bare metal stents (BMS1,2). These findings were reproducible in the most variable and complex scenarios of daily practice3-5.
Together with its many benefits, some concomitant pitfalls of DES include delayed healing and higher rates of late/very late stent thrombosis6-8.
Some studies have suggested that the eluted drug from DES systems might not only affect the stented segment, but also adjacent segments (stent edges) as well9. Previous greyscale intravascular ultrasound (IVUS) analyses have pointed to different vessel responses (arterial remodelling) and plaque growth at proximal and distal edges of BMS and DES10,11.
We speculated that the use of spectral analysis of radiofrequency ultrasound backscatter signals (Virtual Histology® intravascular ultrasound [VH® IVUS]; Volcano Corporation, Rancho Cordova, CA, USA) to analyse plaque composition and modification at the edge segments of BMS and DES could increase our understanding of the differences in the healing process at those segments.
Methods
Study design, population and objectives
This single-centre, prospective and randomised study aimed to assess the differences in artery remodelling and plaque composition at the edges of patients treated with BMS (Driver™; Medtronic, Santa Clara, CA, USA) or DES (Cypher Select™; Cordis, Miami Lakes, FL, USA).
Only patients who initially presented with acute coronary syndrome were included. Furthermore, the culprit lesion had to be located in a native coronary artery of 2.5 to 3.5 mm in diameter and had to be treated with a single stent implantation (up to 33 mm in length). We excluded patients with ST-segment elevation myocardial infarction (MI) treated in the very early phase (primary or rescue percutaneous coronary intervention [PCI]), restenotic lesions, lesions located at grafts and at the left main stem. Patients with planned surgery within one year of the intervention or those with previous history of major bleeding or allergy to aspirin or thienopyridine were excluded as well. From the angiographic point of view, major exclusion criteria were the lack of edge segments (ostial lesions and presence of major side branches within the 5 mm proximal or distal to the stent) and pre-PCI TIMI flow ≤1.
The randomisation was performed using sealed envelopes picked by research staff unaware of the procedure.
Only the culprit lesion was treated in the index procedure. In case of multivessel disease, other lesions were treated in staged procedures within the first month of the baseline procedure.
All patients underwent greyscale IVUS and VH-IVUS assessment immediately after stent deployment and at nine-month invasive follow-up. Through the follow-up period patients followed a standardised protocol, which included office appointment at one, three, six and 12 months and hospitalisation at nine months for invasive follow-up.
The main objectives of the study included the comparison in the variation in vessel, lumen and plaque area at proximal and distal edges of BMS and DES and the comparison in plaque composition at proximal and distal edges of BMS and DES between baseline and the nine-month follow-up period. Additionally, a correlation between the two imaging modalities aimed to determine the association between modification in plaque composition and in vessel geometry.
The study was approved by our local ethics committee and written informed consent was obtained from all patients prior to enrolment. Randomisation to BMS or DES (1:1) was performed immediately after the signature of the informed consent.
Percutaneous coronary intervention
Prior to intervention all patients were pre-treated with aspirin (200 mg) and clopidogrel (600 mg). During the procedure, an intravenous bolus of heparin was given (5000 IU) to keep the activated clotting time (ACT) between 250-350 seconds. Use of other drugs like IIb-IIIa inhibitors was left at the operator’s discretion. Percutaneous coronary intervention was performed according to our institution’s routine and in accordance with international guidelines. However, the use of direct stenting was highly recommended and operators were oriented towards deploying the stents at intermediate pressures (between 10 and 12 atm) followed by high-pressure post-dilatation with shorter non-compliant balloon catheters chosen under IVUS guidance. In all cases we tried to avoid the occurrence of acute stent malapposition as well as gross underexpansion. Whenever possible, we recommended the minimum stent area to be at least 70 to 80% of the mean reference areas. This strategy was intended to minimise stent edge barotrauma, one possible cause of “edge effect”.
At the end of the procedure, a bolus of 100-200 mcg of intracoronary nitroglycerine was given and greyscale IVUS with VH-IVUS were performed. Greyscale and VH-IVUS data were sent to an independent corelab (Cardiovascular Research Center, São Paulo, Brasil).
After the procedure, aspirin (100 mg/day) was prescribed indefinitely and clopidogrel (75 mg/day) for at least 12 months. Unless in the presence of a formal contraindication, beta-blockers, angiotensin-converting-enzyme (ACE) inhibitors and statins were prescribed to all patients, according to current guidelines.
Quantitative coronary angiography (QCA)
Angiographic studies were performed at baseline, post-procedurally and at follow-up, in two orthogonal views, after the intracoronary administration of 100-200 µg of nitroglycerine. The same angiographic angles performed at baseline were reproduced during the subsequent studies. Digital angiograms were analysed off line with the use of an automated edge-detection system (QCA-CMS®; Medis Medical Imaging Systems, Nuenen, The Netherlands). The contrast-filled catheter tip was used for calibration.
Quantitative angiographic parameters at the proximal and distal edge assessment included: 1) reference vessel diameter; 2) minimum lumen diameter (MLD); 3) percent diameter stenosis (difference between the reference diameter and MLD divided by the reference diameter and multiplied by 100); and 5) late luminal loss (difference between MLD at the end of the procedure and MLD at follow-up). Quantitative analysis was performed in the 5 mm proximally and distally to the stent.
Greyscale IVUS
IVUS imaging was obtained with an automatic pullback (R100) at the constant speed of 0.5 mm/sec starting at least 10 mm distal to the stent edge and ending at the coronary ostium. All studies were performed with the 2.9 Fr 20 MHz Eagle-Eye® catheter (Volcano Therapeutics, Rancho Cordova, CA, USA) using a dedicated console. Prior to IVUS imaging acquisition, an intracoronary100-200 mcg of nitroglycerine was given. Analyses were performed by two independent experienced operators blinded to the procedure characteristics, including the type of stent deployed. All analyses were done according to international guideline12.
A dedicated software (echoPlaque® 3.0; INDEC Medical Systems, Santa Clara, CA, USA ) was used for the analysis, which included the measurement of elastic external membrane (EEM) cross-sectional area (CSA) and lumen CSA within 5 mm immediately adjacent to the implanted stent (proximal and distal edges). Plaque CSA was derived from the EEM –lumen CSA and EEM; lumen and plaque volumes were automatically calculated by the software. The same procedure was performed at baseline and follow-up analysis.
All measurements were performed by the two experienced operators blinded to procedure details. Inter and intraobserver variability was determined by Kappa test.
VH-IVUS analysis
VH-IVUS images were generated simultaneously with greyscale IVUS during automatic pullback evaluation of the vessel. Images were acquired at every R-peak during ECG registration. Atheroma composition was based on the previously validated mathematical autoregressive spectral analysis using dedicated software (pcVH 2.2; Volcano Therapeutics, Rancho Cordova, CA, USA). Four components were identified, quantified and colours were attributed to each of them: fibrotic tissue (green), fibrofatty (light green), dense calcium (white) and necrotic core (red).
Measurements were made for the proximal and distal stent edges, which were defined as the 5 mm adjacent to the stent.
All measurements were performed by two experienced operators (different from the ones who analysed greyscale IVUS) blinded to procedure details. Inter and intraobserver variability was also determined by Kappa test. All analyses were performed according to international guidelines13.
Statistical analysis
Categorical data was presented as proportions and compared with chi-square or Fisher’s exact tests. Continuous data was presented as mean±standard deviation. When continuous data was evenly distributed, an independent sample t-test was used. Unevenly distributed data was compared using the non-parametric Mann-Whitney U test. For analysis of continuous data in different time-points (e.g., baseline vs. follow-up), the paired Student’s t test was applied. Correlation between plaque composition and modification in vessel geometry was performed using the Pearson’s test. Inter and intraobserver variability was calculated using the Kappa test. Fifteen randomly selected cross-sectional areas were selected for vessel and lumen contour tracing and necrotic core determination. For intraobserver evaluation, the same cross-sectional areas were measured with an interval of two to three weeks.
All tests were two-sided and a p-value <0.05 was considered significant. All analyses were performed with SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Population characteristics and procedure results
Between September 2008 and September 2009, a total of 48 patients were enrolled. Among them, four refused invasive follow-up, two developed renal insufficiency and were not evaluated invasively (both were asymptomatic) and in two cases image quality was deemed inappropriate to analysis due to problems with pullback speed. Therefore, a total of 40 patients (20 treated with BMS and 20 with DES) were included in the final analysis.
There was no significant difference between the groups (Table 1). Overall, patients were relatively young (~56 years old), predominantly male (70%) with a high prevalence of diabetes, hypertension and smoking. Sixty percent of the patients in each group had positive troponin at the time of enrolment. Initial clinical presentation most frequently encountered was ST-elevation MI (>72 h of the onset).
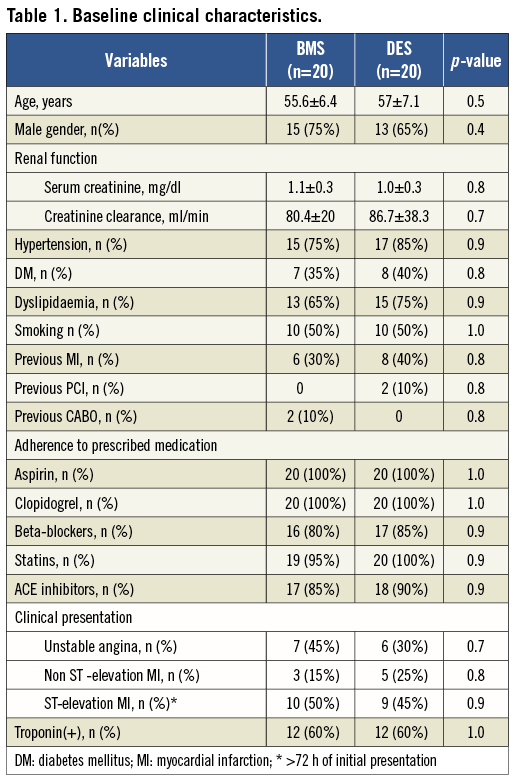
Procedure data is shown in Table 2. Again, no significant difference was observed. Mean time between onset of symptoms and PCI was about 13 days for patients in the BMS cohort vs. 11.8 days for those in the DES group (p=0.32). The vast majority of patients in both groups had a single lesion and the left anterior descending artery (LAD) was the most frequently treated artery. Final TIMI 3 flow was achieved in all patients. Direct stenting was achieved in >60% of all cases. Following the proposed strategy, average stent deployment pressure was 10.3±1.9 atm for the BMS group and 11.1±1.6 atm for the DES group (p=0.23). Post-dilatation with a high pressure balloon was performed in hardly any patients (mean pressure ~16 atm). IIb-IIIa inhibitors were not used among these patients.
Angiographic success was achieved in all cases and patients stayed 1.4 days in the hospital after the procedure.
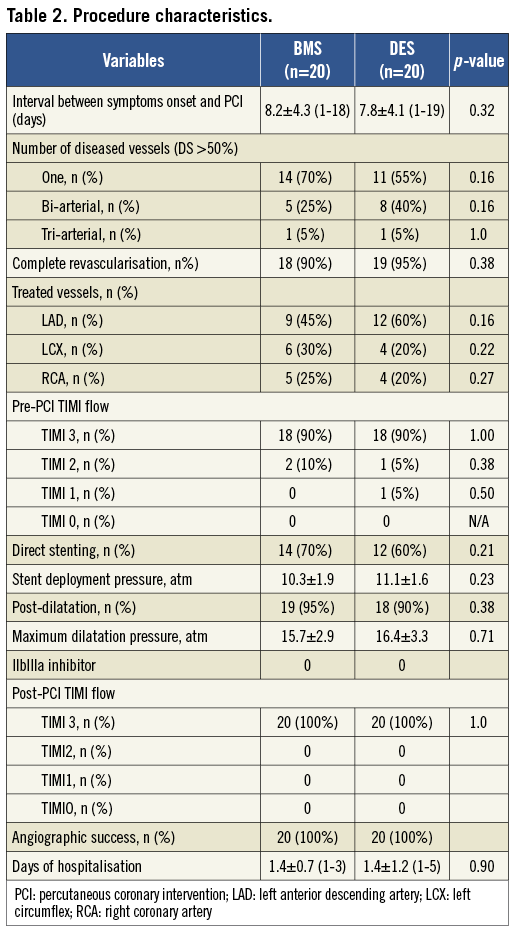
Quantitative angiography results
Table 3 summarises the main QCA findings at the proximal and distal edges of BMS and DES. For both stents, reference vessel diameter was bigger at proximal than distal edges. Late lumen loss was non-significantly higher at the proximal edges of patients treated with BMS as compared to those who received a DES (0.28±0.25 mm vs. 0.20±0.07 mm, p=0.1). At the distal edge, there was a significantly more pronounced reduction in late loss among patients who received DES (0.20±0.11 mm vs. 0.38±0.25 mm, p=0.009).
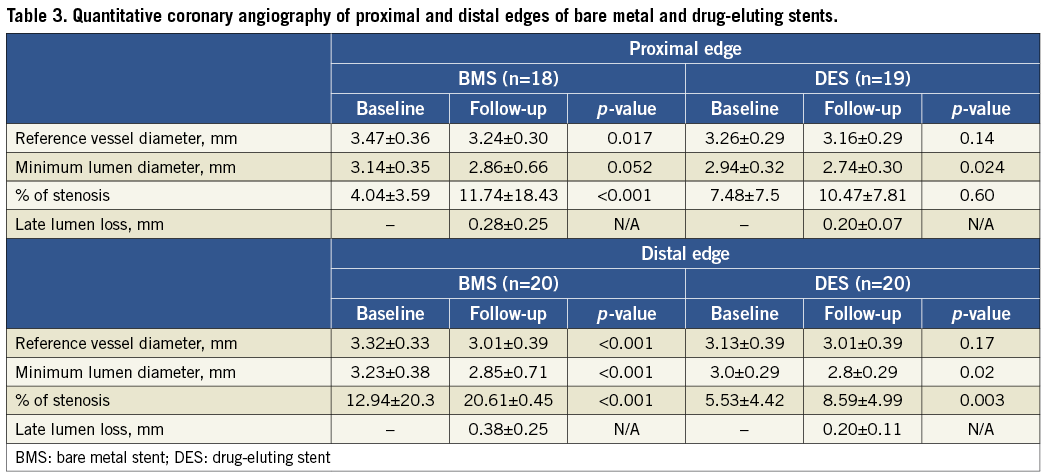
Greyscale IVUS results
Table 4 displays the variation in mean vessel, lumen and plaque areas from baseline to follow-up at proximal and distal edges of BMS and DES. Of note, there was a trend for positive vessel remodelling at the proximal edge of patients treated with DES (∆=+0.6 mm, p=0.06). No major changes were observed at the proximal edge of BMS. Figure 1 shows the comparison of the area variation for BMS and DES at the proximal edge.
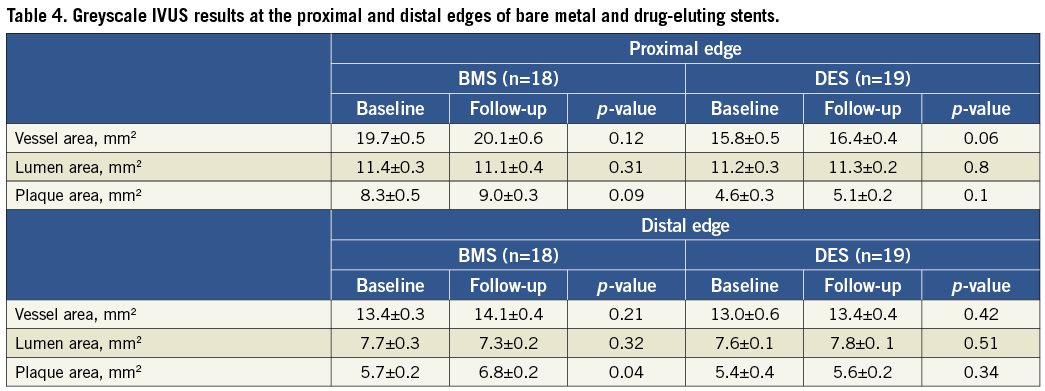
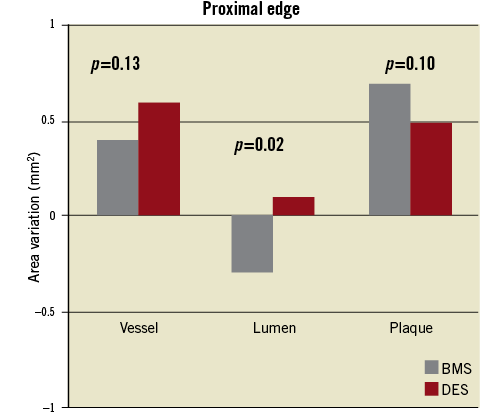
Figure 1. Comparison of mean vessel, lumen and plaque area changes at the proximal edge of bare metal and drug-eluting stents.
A different response was seen at the distal edge of the stents. At that site, BMS showed a more pronounced vessel enlargement (∆=+0.7 mm vs. +0.4 mm, p=0.013) as compared to DES. However, plaque growth was also markedly superior among patients treated with BMS (∆=+1.1 mm vs. +0.2 mm, p<0.001). As a consequence, there was a slight increase in lumen area in the DES group versus a more significant lumen diminishing among those who received a BMS (∆=+0.2 mm vs. –0.4 mm, p=0.022). Figure 2 shows the comparison between DES and BMS regarding vessel, lumen and plaque variations.
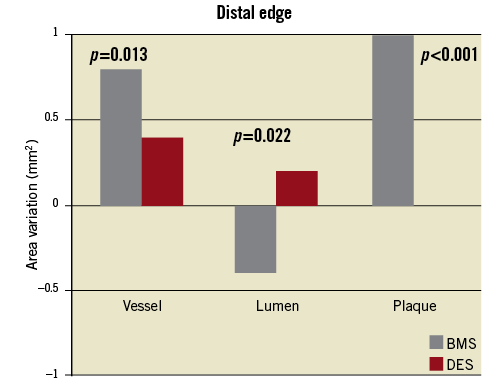
Figure 2. Comparison of mean vessel, lumen and plaque area changes at the distal edge of bare metal and drug-eluting stents.
Figure 3 shows the pattern of geometrical vascular response for BMS and DES every 1 mm within the proximal and distal edges. Interestingly, most of the meaningful changes in the lumen area occurred within the first 3 mm of the distal segment.
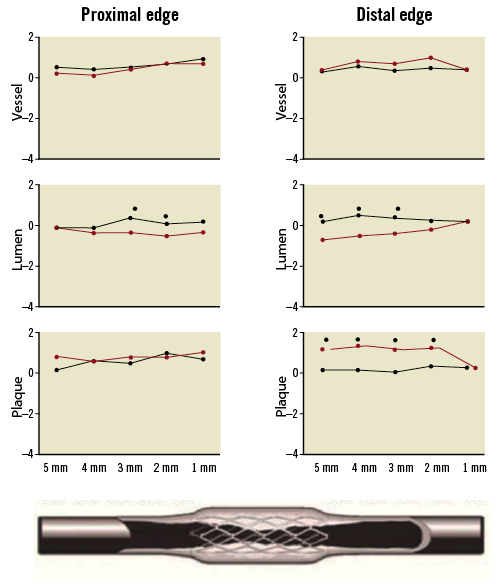
Figure 3. Sub-segment (each 1.0 mm) changes at the proximal and distal edges of bare metal and drug-eluting stents.
Kappa tests for inter and intraobserver variability in measuring the smallest lumen area at the proximal and distal reference segments were 0.90 and 0.93, respectively.
VH-IVUS results
Table 5 summarises the main findings regarding plaque composition at baseline and follow-up. For both types of stents there was a predominance of fibrotic tissue followed by necrotic core, dense calcium and fibrofatty tissue at the edges.
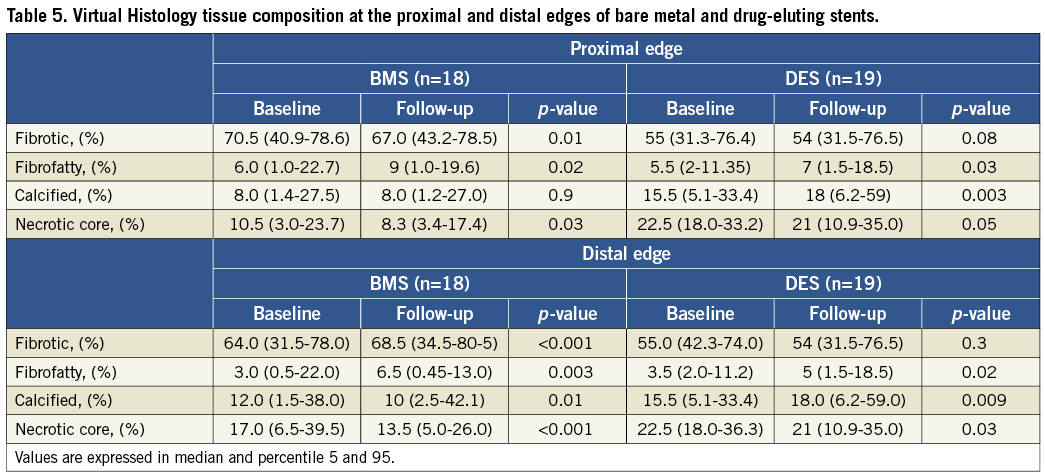
Proximal edge
Among patients treated with BMS there was a significant decrease in the amount of fibrotic tissue (∆=–3.5%, p=0.01) and necrotic core (∆=–2.2%, p=0.03) in the follow-up period. Conversely, there was a significant increase in the percentage of fibrofatty tissue (∆=+3.0%, p=0.03) while the percentage of dense calcium did not change. Patients treated with DES also experienced a reduction in the percentage of fibrotic tissue (∆=–1.0%, p=0.08), and necrotic core
(∆=–1.5%, p=0.05) at the proximal reference segment. In this cohort, there was a significant increment in the percentage of fibrofatty tissue (∆=+3.5%, p<0.001) and dense calcium (∆=+2.5%, p=0.003).
Distal edge
The amount of each component followed the same sequence as the proximal edge, the fibrotic tissue being the most prevalent component for both stents. Patients treated with BMS had a significant increase in the percentage of fibrotic (∆=+ 4.5%, p<0.001)and fibrofatty tissue (∆=+1.5%, p=0.02) while necrotic core (∆=–3.5%, p<0.001) and dense calcium (∆=–2.0%, p=0.01) were reduced. Patients treated with DES did not show a marked variation in the percentage of fibrotic tissue (∆=–1.0%, p=0.3) but showed a significant increase in percentage of dense calcium (∆=+2.5%, p=0.009) and fibrofatty tissue (∆=+3.5%, p<0.001). Conversely, the percentage of necrotic core was markedly reduced (∆=–1.5%, p=0.03).
Person´s correlation tests showed no association between plaque composition at the time of the procedure and the modifications observed in the vessel geometry at the edges of BMS and DES (Table 6). However, the assessment of the correlation between variations in plaque composition and modification in vessel geometry pointed to a strong correlation between increase of fibrofatty component and growth of the plaque area (r=0.78, p=0.01). Variation in the amount of the other components did not correlate with any other vessel, lumen or area change (Table 7).
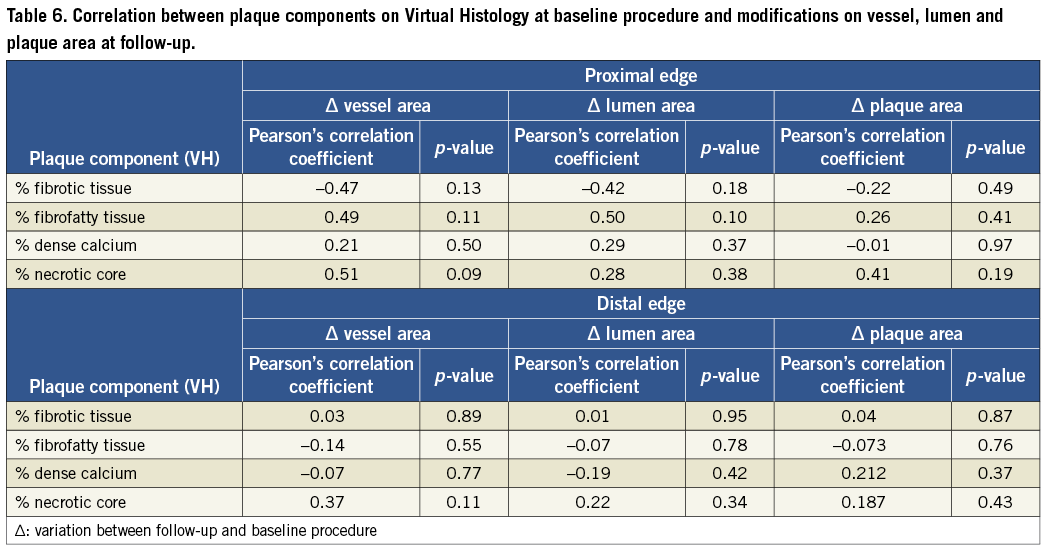
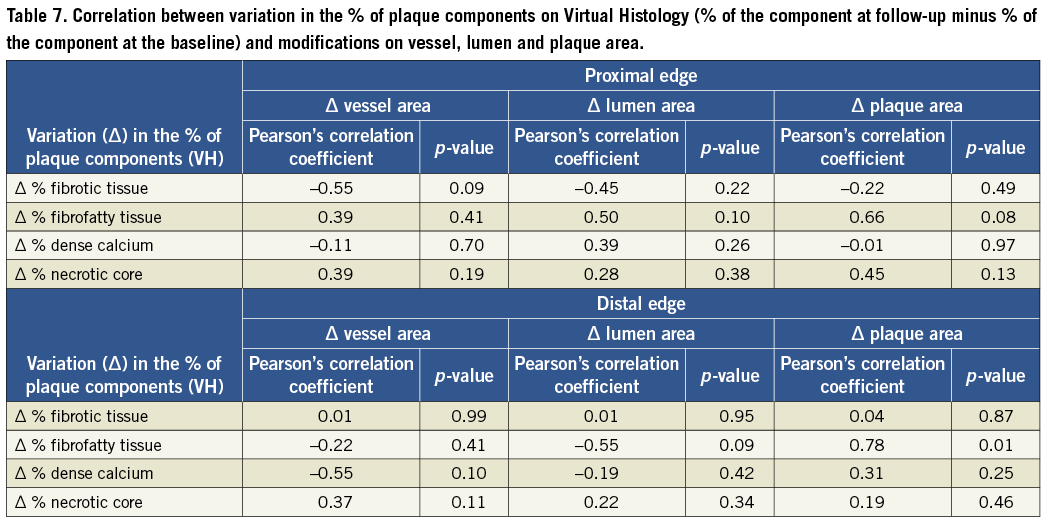
Kappa tests for inter and intraobserver variability in determining the percentage of necrotic core in the proximal and distal reference segments for VH-IVUS analysis were 0.84 and 0.88, respectively.
Clinical outcomes
At the end of one year, there were no cases of death, MI, stroke or stent thrombosis. However, five patients (12.5%) presented with restenosis requiring another PCI. There were four cases of in-stent restenosis (all in the BMS cohort) and one case of (distal) edge restenosis (a patient enrolled in the DES cohort).
Additionally, two patients required non-target vessel PCI due to lesion progression.
Discussion
The main findings of the present analysis were: 1) at the proximal edge of the vessel treated with a first generation DES there was a trend toward positive vessel remodelling; 2) while at the distal edge, less plaque growth was observed resulting in larger lumen area at follow-up; 3) at the proximal edge of both stents there was a marked increase of fibrofatty component in the follow-up accompanied by a reduction in fibrotic tissue and necrotic core; 4) at the distal edge there was a marked increase in fibrofatty component among patients treated with BMS, while in the DES cohort, there was an increase of the percentage of dense calcium; and (5) a strong positive correlation between increment in fibro-fatty tissue (VH-IVUS) and increase in plaque area (greyscale IVUS).
Restenosis is a healing response to local vessel injury and manifests itself mainly by vessel (negative) remodelling and excessive neointimal formation14. While neointimal proliferation follows a temporal cascade of events including platelet aggregation, local inflammatory cell infiltration and proteoglycan deposition15, little is known about vessel remodelling. This term is used to address vessel modifications that result in expansion of arterial lumen (positive remodelling) and also vessel shrinkage (negative remodelling). Negative vessel remodelling seems to be more related to the occurrence of restenosis and among the involved mechanisms in its occurrence, deposition of myofibroblasts seems to have a central role in the process16,17.
While the advent of bare metal stents more or less eliminated negative vessel remodelling18, the presence of a “foreign metallic body” inside the coronary together with the barotrauma caused by the inflation pressure in deploying the stent resulted in a more exacerbated arterial wall inflammatory response, leading to a reparatory process, which in some cases might result in excessive neointimal tissue formation and restenosis19.
To prevent restenosis, many drugs were locally and systemic tested as well as novel mechanical devices20-22. Among them, only brachytherapy showed initial promising results, with important attenuation of neointimal tissue formation23,24. However, initial enthusiasm was lessened by the occurrence of edge restenosis (dubbed “edge effect”) and late recurrence of in-stent restenosis (dubbed “latch catch-up”)25-27.
The use of drug-eluting stents, that act directly in the cell cycle, was aimed at preventing, in a predictable and sustained way, the hyperproliferative response to artery wall injury. While they were showed to be very effective in reducing in-stent neointimal tissue growth, first reports of edge restenosis prompted the debate about edge effect.
Intravascular ultrasound remains the best imaging modality to evaluate in vivo changes in vessel geometry. Modifications in stent edges have been previously assessed in other studies. For instance, Asano et al evaluating 30 patients (33 lesions) treated with Cypher™ noted a plaque growth at both stent edges, with maintenance of vessel area resulting in reduction of lumen size. Plaque growth was more pronounced in the first millimetre from each edge10.
Serruys et al, using similar methodology but evaluating the TAXUS® paclitaxel-eluting stent (Boston Scientific, Natick, MA, USA), compared the vessel modifications at the edges of these stents in comparison to the bare metal equivalent (Liberté®; Boston Scientific)11. In this sub-analysis of the TAXUS II trial 106 patients treated with the slow-release formulation of paclitaxel were included, 107 with the moderate release and 214 with the BMS. The main findings included reduction in mean lumen area of the three stents at the proximal stent edge, while at the distal edge only patients treated with BMS had reduction of the lumen (due to less plaque growth in the DES groups). Additionally, negative vessel remodelling was observed at the proximal but not at the distal edges of all three stents.
We note therefore that modifications in vessel, lumen and plaque area may vary from study to study, according to the type of stent evaluated and the edge analysed. Other characteristics that may also account for these distinct results include: 1) residual plaque burden at the edges of stents at the baseline procedure, which is an independent IVUS predictor of both stent thrombosis and restenosis; 2) vessel tortuosity and shear-stress; 3) plaque composition at stent edges and its modification over time; 4) adherence to prescribed medical therapy, especially statins, which have been shown not only to stop plaque growth but also to change its composition.
More recently, the advent of radiofrequency IVUS allowed us not only to evaluated changes in vessel geometry but also to access plaque composition modifications at the same segments.
Two recent publications used this imaging tool to evaluate the changes at the edges of DES. In the BETAX (BEside TAXus) trial, Garcia-Garcia et al evaluated 24 patients treated with the TAXUS® stent28. Their main findings included: 1) the occurrence of positive vessel remodelling at the proximal edges of the stents compensating plaque growth at that segment and preserving the lumen; 2) at the distal edge, positive vascular remodelling in combination with less plaque growth resulted in increase in lumen area; 3) by VH-IVUS the authors observed a significant reduction in the percentage of dense calcium (∆=–4,9% at the proximal edge, p<0.001; e ∆=– 7.7%, p<0.001 at the distal edge) and necrotic core (∆=–2.8% at the proximal edge, p=0.002; e ∆=–7.7% at the distal edge, p<0.001) between baseline and six-month follow-up at both edges. Conversely, the fibrofatty component markedly increased at both edges (∆=+7.0% at the proximal edge, p<0.001; e ∆=+ 6.7% at the distal edge, p<0.001). The authors did not correlate greyscale IVUS and VH-IVUS findings.
In the sub-analysis of the MISSION study, Atary et al compared changes in vessel geometry and plaque composition at the edges of BMS and Cypher™ in 40 patients (20 in each group) treated with primary angioplasty for ST-elevation MI29. The main findings of the study included similar behaviour between BMS and DES at the proximal edge and a trend toward negative vessel remodelling in the BMS cohort at the distal edge (∆ vessel volume=–5.5 mm3, p=0.09), while patients treated with the Cypher™ stent tended to present with expansive vessel remodelling (∆ vessel volume= +1.8 mm3, p=0.21). The authors did not observe significant modifications in plaque composition in this study. Again, the findings of the studies are not homogeneous and the differences might be attributed to the type of patients evaluated, the type of stents deployed and the way the stents were deployed.
Although only speculative and hypothesis-generating, the fact that we recommended stent deployment at low/intermediate pressures (on average <12 atm) followed by high pressure post-dilatation with shorter non-compliant balloons, might have influenced the results since there might be an attenuation of the barotrauma at stents edges. In 2008, Costa et al reported the results of the multicentre and prospective STLLR trial that evaluated the frequency and impact of suboptimal percutaneous coronary interventions on long-term outcomes of 1,557 patients treated with sirolimus-eluting stents in US hospitals. They sought to determine the impact of geographical miss (longitudinal and axial) in the outcomes of complex patients treated with PCI. Geographical miss occurred in 66.5% of all cases and longitudinal miss (injured or diseased segments not covered by the stent) was the most frequent (47.6% had longitudinal, 35.2% had axial, and 16.5% had both). Notably, the occurrence of geographical miss resulted in an increase in target lesion revascularisation (TLR) and MI rates30. In the present study, the strategy to perform PCI might have minimised the occurrence of this adverse complication.
Limitations
Some significant limitations of this research should be acknowledged. First, the relatively small sample size enrolled. Since this is an exploratory analysis, there was no formal statistical hypothesis. Also, the relatively complex design of the trial and the clinical profile of the included patients precluded a broader enrolment. Therefore, the present findings should be validated in a larger and more complex cohort.
Another potential limitation refers to the time interval between the baseline and follow-up examination (nine months), which may be a short time to assess modifications in plaque composition.
Finally, there are some intrinsic limitations of VH-IVUS that should be pointed out, such as lack of proper tissue characterisation for thrombus, a relatively frequent finding among these patients. As a consequence, measurement errors cannot be disregarded.
Conclusions
The use of DES in patients with acute coronary syndrome did not result in “edge effect” among the studied population. Conversely, the use of this novel technology leads to less plaque growth at the distal stent edge, which may reflect the “wash-out” effect of the drug by the coronary blood flow. Overall, patients treated with DES or BMS had a decrease of fibrotic and necrotic core plaque components at both edges between index procedure and nine-month follow-up. Importantly, variations in the percentage of fibrofatty component directly correlated with plaque area growth.
The role of the statins and the technique for deploying the stent should be evaluated in larger studies designed for that purpose.
Funding
The stents used in this study were donated by Cordis Corporation to our institution.
Conflict of interest statement
The authors have no conflicts of interest to declare.

