Abstract
Background: The integrative implications of quantitative and qualitative plaque characteristics on clinical outcomes and therapeutic guidance have not been fully investigated.
Aims: We aimed to investigate the combined prognostic value of quantitative and qualitative plaque measures and their interactions with treatment modalities and physiological lesion severity.
Methods: Among 697 vessels from 458 patients who underwent fractional flow reserve (FFR)-guided treatment, quantitative high-risk plaque (qn-HRP; plaque burden ≥70% and minimum lumen area <3.3 mm2) and qualitative HRP (ql-HRP; low-attenuation plaque or positive remodelling) were defined on coronary computed tomography angiography (CCTA). The primary endpoint was the vessel-oriented composite outcome (VOCO; a composite of cardiac death, myocardial infarction, or revascularisation).
Results: The mean baseline FFR was 0.85±0.12, and 25.8% underwent percutaneous coronary intervention (PCI) during the index procedure. In medically treated lesions, both qn-HRP and ql-HRP were associated with an increased risk of VOCO (p<0.05). Relative to the lesions with qn-HRP(-)/ql-HRP(-),those with qn-HRP(+)/ql-HRP(+) showed a higher risk of VOCO (hazard ratio [HR] 8.36, 95% confidence interval [CI]: 2.86–24.44). The PCI group showed a lower risk for VOCO than the medical treatment group (HR 0.31, 95% CI: 0.11–0.91) in lesions with qn-HRP(+)/ql-HRP(+). This difference was consistent in lesions with an FFR of 0.81–0.90 (HR 0.19, 95 CI: 0.04–0.90), but not in those with an FFR of>0.90.
Conclusions: In non-ischaemic lesions, ql-HRP and qn-HRP showed a synergistic impact on risk assessment and had prognostic interactions with FFR and treatment modalities. Therefore, they need to be integrated into risk stratification and the optimisation of a treatment strategy. ClinicalTrials.gov: NCT04037163.
Introduction
Coronary artery disease (CAD) can be assessed based on anatomical, morphological, and physiological attributes. Myocardial ischaemia, as verified by invasive physiological indices such as fractional flow reserve (FFR), has been used as an indicator for revascularisation12. Nonetheless, clinical events still occur after FFR-guided deferral of percutaneous coronary intervention (PCI)3, and recent studies have suggested that revascularisation based on myocardial ischaemia might not warrant favourable outcomes in comparison with optimal medical treatment45.
Coronary plaque characteristics are also associated with the risk of coronary events. Various plaque features reflective of their quantity and quality have been identified67891011 and have been reported to be independent predictors of clinical events, even in non-ischaemic lesions12131415. Moreover, the additive prognostic value of quantitative and qualitative plaque characteristics has been suggested1116. Nonetheless, there is no established consensus on the indications for plaque characteristics to warrant PCI. Thus, which component is the main driver of clinical events in non-ischaemic lesions and whether it has a synergistic impact on clinical outcomes and therapeutic guidance needs to be elucidated.
In this regard, we aimed to investigate the individual and combined prognostic implications of coronary computed tomography angiography (CCTA)-derived quantitative and qualitative plaque metrics in non-ischaemic lesions, as well as their prognostic interactions with treatment modalities and physiological stenosis severity.
Methods
Study participants
The study population was from the CCTA-FFR Registry (ClinicalTrials.gov: NCT04037163), the profile of which has been previously published in detail17. Briefly, the CCTA-FFR Registry included patients with suspected CAD who had undergone invasive FFR measurement and CCTA within 90 days before the FFR measurement. This registry was used to evaluate the prognostic implications of both the physiological and morphological aspects of coronary lesions. All treatment decisions were made at the physician’s discretion. Patients with a depressed ejection fraction (<35%), acute ST-elevation myocardial infarction (STEMI) within 72 hours, history of coronary artery bypass graft surgery, chronic kidney disease, Thrombolysis in Myocardial Infarction flow <3, or planned coronary artery bypass graft surgery after angiography were excluded. The current study included 697 vessels from 458 patients who underwent FFR-guided treatment. For revascularised low-FFR (≤0.80) lesions, only cases with a post-PCI FFR>0.80 were included. The study protocol was approved by the institutional review board of each participating centre.
Invasive coronary angiography, FFR measurement and treatment type
Invasive coronary angiography was performed using the standard techniques. Quantitative coronary angiography was performed in optimal projections using a validated software program (CAAS II; Pie Medical Imaging) in an independent core laboratory (Seoul National University Hospital, Seoul, Republic of Korea). FFR measurements were performed using a standard method18. After the engagement of a 5 to 7 Fr guide catheter, the pressure-temperature sensor guidewire was set to 0 and equalised to the aortic pressure. A continuous intravenous infusion of adenosine (140 μg/kg/min) or adenosine 5'-triphosphate (ATP; 160 μg/kg/min) was used to induce hyperaemic conditions. During hyperaemia, FFR was determined by dividing the mean distal coronary arterial pressure by the aortic pressure. In cases of PCI, post-PCI FFR was measured and designated as the FFR value of the corresponding vessel. All FFR traces were collected and validated in a blinded manner at an independent core laboratory (Seoul National University Hospital, Seoul, Republic of Korea).
Quantitative and qualitative high-risk plaque
Quantitative and qualitative plaque measurements were obtained using CCTA in an independent core laboratory (Severance Cardiovascular Hospital, Seoul, Republic of Korea). All CCTA procedures were performed according to the Society of Cardiovascular Computed Tomography guidelines19. Semi-automated software (QAngioCT Research Edition, version 2.1.9.1; Medis) with appropriate manual correction was used for qualitative and quantitative plaque analysis in CCTA. Intraobserver, interobserver, and interscan reproducibility of plaque analysis have been previously demonstrated820. If more than one lesion existed in a vessel, the most stenotic lesion was regarded as the representative lesion of the vessel, and its plaque characteristics were analysed.
Plaque burden was defined as the percentage value estimated by the plaque area divided by the vessel area at the minimum lumen area (MLA) segment. Volumetric plaque quantification of a lesion identified the total plaque volume, non-calcified plaque volume (≤130 Hounsfield units), and low-attenuation plaque volume (LAP; ≤30 Hounsfield units). For quantitative plaque measurements, plaque burden and the MLA were used611. Based on the optimal cut-off, derived from Youden’s index in receiver operating characteristic curve analysis, to define the best quantitative metric, plaque burden and MLA were converted into binary variables (i.e., plaque burden ≥70% and MLA <3.3 mm2) (Supplementary Figure 1). With respect to qualitative plaque measurements, 4 adverse plaque characteristics were analysed: LAP (a plaque with a pixel count of ≤30 Hounsfield units), positive remodelling (PR; remodelling index ≥1.1), spotty calcification (SC; the presence of focal calcification with a diameter of <3 mm in any direction), and napkin-ring sign (NRS; ring-like attenuation form with peripheral high and central lower attenuation portion)21. To identify the best quantitative and qualitative metrics for the prediction of the primary outcome, information gain of all possible combinations of binary plaque burden and MLA for quantitative high-risk plaque (qn-HRP) and of 4 adverse plaque characteristics for qualitative high-risk plaque (ql-HRP) were compared in the medical treatment group, and those with the highest information gain were defined as qn-HRP and ql-HRP.
Clinical outcomes
Clinical outcomes were traced in an outpatient clinic or via telephone contact. The primary outcome was the vessel-oriented composite outcome (VOCO), which included cardiac death, target vessel myocardial infarction (MI), or ischaemia-driven target vessel (-related) revascularisation12. All definitions of clinical outcomes were in accordance with the Academic Research Consortium. MI was defined according to the third universal definition of MI2223. Periprocedural MI was not included in the primary outcomes. All clinical events were adjudicated by an independent clinical event committee. Members of the committee received the medical records of clinical events blinded to CCTA findings and physiological data and adjudicated all the clinical events and their causes.
Statistical analysis
All analyses were performed on a per-vessel basis. Continuous variables and categorical variables are presented as mean±standard deviation and number (percentage), respectively. Information gain was used to define the best qualitative and quantitative plaque metrics. Plaque measures with a higher value of information gain are considered more important in the prediction of VOCO. Using a bootstrapping method with 10,000 replicates, the mean value and 95% confidence interval (CI) of each measure’s information gain were calculated and presented. To evaluate the improvement in discrimination ability between qn-HRP and ql-HRP, the category-free net reclassification index (NRI) and relative integrated discrimination improvement (IDI) were assessed. The trend in the proportion was assessed using the chi-square test for trend. To compare vessel-specific clinical outcomes, marginal Cox proportional hazard regression was applied to calculate the hazard ratio (HR) and its corresponding 95% CI by accounting for a per-vessel correlation within the same patient. The individual patient was specified to evaluate the robust sandwich variance estimates of the coefficients. In the multivariate analyses, clinical characteristics, medication history, and lesion characteristics that differed between the medical treatment and PCI groups were included as covariates. These were included in separate models to avoid overfitting. P-values <0.05 were regarded as statistically significant. All analyses were performed using R (version 4.1.1; R Foundation for Statistical Computing).
Results
Baseline characteristics and identification of qn-HRP and ql-HRP
Table 1 shows the baseline patient and lesion characteristics. The mean age was 65.7±9.8 years, and 72.1% were male. The mean % diameter stenosis and baseline FFR were 45.5±17.2% and 0.85±0.12, respectively, and 25.8% underwent PCI during the index procedure. The overall cumulative incidence of VOCO was 7.2% (24 events) in the medical treatment group and 9.1% (12 events) in the PCI group. The individual components of VOCO are presented in Supplementary Table 1. The PCI group had a higher proportion of men, hyperlipidaemia, and acute coronary syndrome than the medical treatment group. Aspirin, P2Y12 inhibitors, and statins were prescribed more frequently in the PCI group than in the medical treatment group. Supplementary Figure 2 represents the relative importance of the combination of quantitative and qualitative plaque measures in the prediction of VOCO. The presence of both a plaque burden ≥70% and an MLA <3.3 mm2 showed the highest information gain and was defined as qn-HRP. Among the combinations of 4 adverse plaque characteristics, the presence of LAP or PR showed the highest information gain and was defined as ql-HRP.
Table 1. Baseline characteristics.
| Total | Medical treatment | PCI | p-value | ||
|---|---|---|---|---|---|
| Patients | N=458 | N=290 | N=168* | ||
| General characteristics | Age (years) | 65.7±9.8 | 66.3±9.8 | 64.8±9.9 | 0.115 |
| Male | 330 (72.1) | 194 (66.9) | 136 (81.0) | 0.002 | |
| Cardiovascular risk factors | Hypertension | 312 (68.1) | 193 (66.6) | 119 (70.8) | 0.399 |
| Diabetes mellitus | 163 (35.6) | 102 (35.2) | 61 (36.3) | 0.886 | |
| Hyperlipidaemia | 268 (58.5) | 158 (54.5) | 110 (65.5) | 0.028 | |
| Current smoker | 105 (22.9) | 61 (21.0) | 44 (26.2) | 0.250 | |
| Clinical presentations | Stable coronary artery disease | 373 (81.4) | 24 8 (85.5) | 125 (74.4) | 0.005 |
| Acute coronary syndrome (unstable angina or NSTEMI) | 85 (18.6) | 42 (14.5) | 43 (25.6) | ||
| Discharge medication | Aspirin | 365 (79.7) | 209 (72.1) | 156 (92.9) | <0.001 |
| P2Y12 inhibitor | 283 (61.8) | 129 (44.5) | 154 (91.7) | <0.001 | |
| ACEi/ARB | 215 (46.9) | 132 (45.5) | 83 (49.4) | 0.480 | |
| Beta blocker | 148 (32.3) | 95 (32.8) | 53 (31.5) | 0.870 | |
| Calcium channel blocker | 207 (45.2) | 136 (46.9) | 71 (42.3) | 0.388 | |
| Statin | 371 (81.0) | 223 (76.9) | 148 (88.1) | 0.005 | |
| Lesions | N=697 | N=517 | N=180 | ||
| Vessel | Left anterior descending artery | 326 (46.8) | 200 (38.7) | 126 (70.0) | <0.001 |
| Left circumflex artery | 158 (22.7) | 138 (26.7) | 20 (11.1) | ||
| Right coronary artery | 213 (30.6) | 179 (34.6) | 34 (18.9) | ||
| Diameter stenosis, % | 45.5±17.2 | 40.0±15.0 | 61.5±12.4 | <0.001 | |
| FFR† | 0.85±0.12 | 0.90±0.06 | 0.68±0.11 | <0.001 | |
| FFR§ | 0.90±0.06 | 0.90±0.06 | 0.89±0.05 | 0.001 | |
| Quantitative plaque features | Total plaque volume, mm3 | 140.2±138.7 | 125.8±136.0 | 181.7±138.3 | <0.001 |
| Non-calcified plaque volume, mm3 | 28.7±46.9 | 23.0±41.8 | 44.9±56.1 | <0.001 | |
| Low-attenuation plaque volume, mm3 | 3.1±8.9 | 2.2±6.4 | 5.5±13.4 | 0.002 | |
| HRP features | MLA <3.3 mm2 | 375 (53.8) | 226 (43.7) | 149 (82.8) | <0.001 |
| Plaque burden ≥70% | 259 (37.2) | 147 (28.4) | 112 (62.2) | <0.001 | |
| Low-attenuation plaque | 134 (19.2) | 71 (13.7) | 63 (35.0) | <0.001 | |
| Positive remodelling | 282 (40.5) | 195 (37.7) | 87 (48.3) | 0.016 | |
| qn-HRP and ql-HRP¶ | None | 261 (37.4) | 224 (43.3) | 37 (20.6) | <0.001 |
| Either | 298 (42.8) | 232 (44.9) | 66 (36.7) | ||
| Both | 138 (19.8) | 61 (11.8) | 77 (42.8) | ||
| Values are presented as n (%) for categorical variables and mean±SD for continuous variables. *In the description of patient characteristics, patients who had both medically treated lesions and revascularised lesions were assigned to the PCI group. †pre-PCI FFR in case of PCI. §post-PCI FFR in case of PCI. ¶qn-HRP: MLA <3.3 mm2 and plaque burden ≥70.0%, ql-HRP: low-attenuation plaque or positive remodelling. ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; FFR: fractional flow reserve; HRP: high-risk plaque; MLA: minimum lumen area; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; ql-HRP: qualitative HRP; qn-HRP: quantitative HRP | |||||
Prognostic implications of qn-HRP and ql-HRP in non-ischaemic lesions
In the medical treatment group, the presence of qn-HRP (HR 3.73, 95% CI: 1.63–8.52; p=0.002) or ql-HRP (HR 2.53, 95% CI: 1.03–6.20; p=0.042) was associated with an increased risk of VOCO (Figure 1). When lesions were divided into 4 groups based on qn-HRP and ql-HRP, the risk of VOCO was significantly higher in lesions with both qn-HRP and ql-HRP than in those without qn-HRP and ql-HRP (HR 8.36, 95% CI: 2.86–24.44; p<0.001). However, the lesions with either qn-HRP or ql-HRP showed a similar risk to those with neither (Figure 1). The addition of qn-HRP to ql-HRP significantly improved the NRI and IDI in the prediction of VOCO and vice versa (Supplementary Table 2).
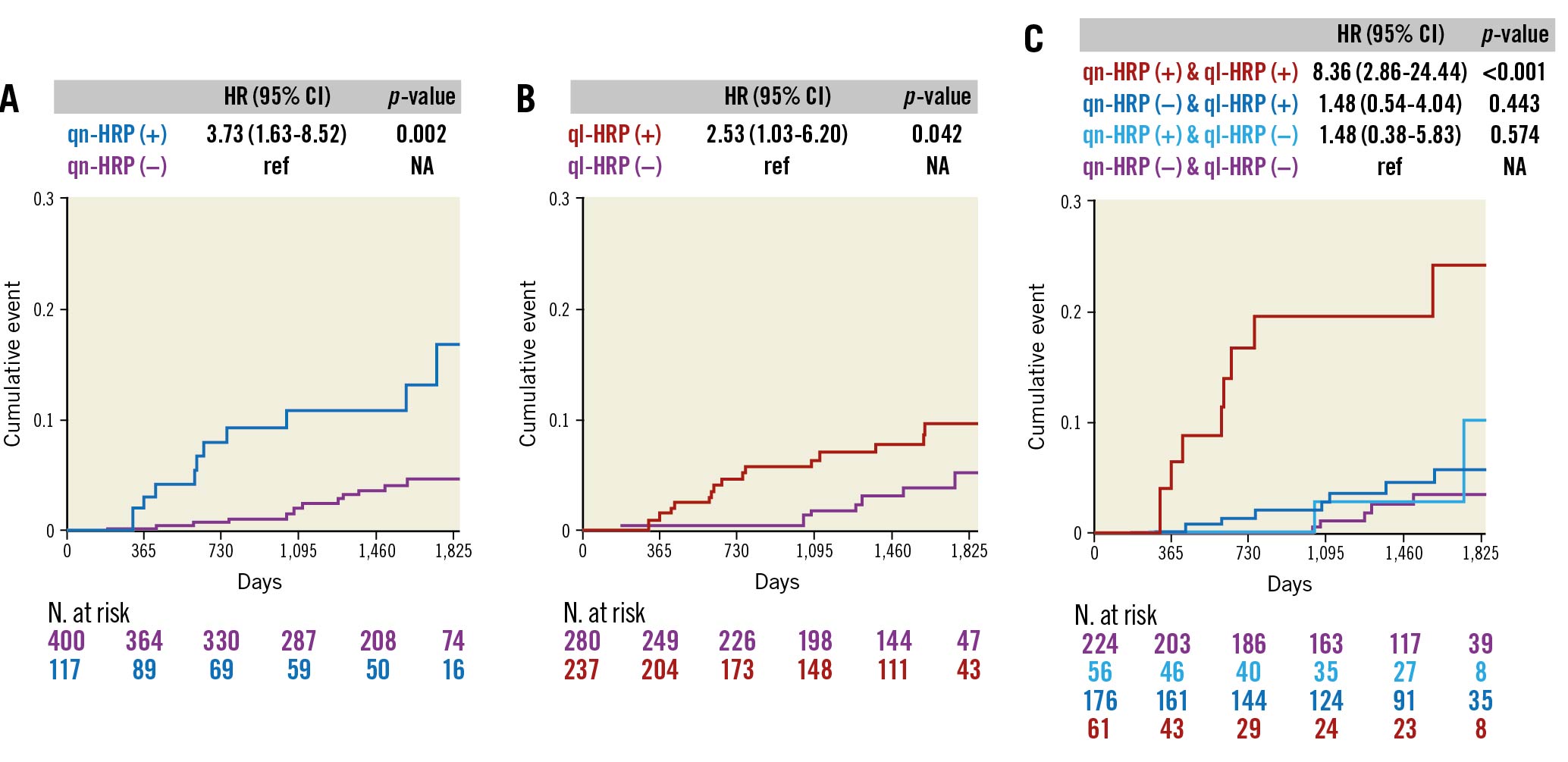
Figure 1. Cumulative events of VOCO according to qn-HRP and ql-HRP in the medical treatment group. A) qn-HRP and B) ql-HRP were associated with an increased risk of VOCO in the medical treatment group with FFR >0.80. C) When lesions were divided into 4 groups based on qn-HRP and ql-HRP, the cumulative event of VOCO was higher only in lesions with qn-HRP (+)/ql-HRP (+) than in those with qn-HRP (–)/ql-HRP (–). qn-HRP was defined as a lesion with an MLA <3.3 mm2 and plaque burden ≥70.0%, and ql-HRP as with low-attenuation plaque or positive remodelling. CI: confidence interval; HR: hazard ratio; HRP: high-risk plaque; NA: not available; ql-HRP: qualitative HRP; qn-HRP: quantitative HRP; VOCO: vessel-oriented composite outcomes
Outcomes with FFR-guided medical treatment and PCI according to HRP
The proportions of none, either, and both qn-HRP and ql-HRP were 43.3%, 44.9%, and 11.8% in the medical treatment group, and 20.6%, 36.7%, and 42.8% in the PCI group, respectively. Supplementary Figure 3 displays the different outcome trends between the medical treatment and PCI groups according to the presence of HRP. In lesions with none, either, or both qn-HRP and ql-HRP, the event rates were 2.7%, 3.9%, and 14.8%, respectively, in the medical treatment group (p for trend=0.001), but were 8.1%, 6.1%, and 6.5%, respectively, in the PCI group (p for trend=0.794). The results were similar after adjusting for clinical characteristics, medications, and lesion characteristics (Table 2). Figure 2 shows the outcome of the medical treatment and PCI groups in each category of none, either, or both qn-HRP and ql-HRP. As compared with the medical treatment group, the PCI group showed a better outcome in lesions with both qn-HRP and ql-HRP (HR 0.31, 95% CI: 0.11–0.91; p=0.033), while not in those with none (HR 3.05, 95% CI: 0.78–12.01; p=0.110) or either (HR 1.36, 95% CI: 0.42–4.37; p=0.604) qn-HRP and ql-HRP. The PCI group showed a lower risk of VOCO in lesions with both qn-HRP and ql-HRP after adjusting for covariates (Table 3).
Table 2.
| Unadjusted HR (95% CI)* | p-value | Model 1 HR (95% CI)* | p-value | Model 2 HR (95% CI)† | p-value | Model 3 HR (95% CI)§ | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Medical treatment group | |||||||||
| qn-HRP and ql-HRP | None | Reference | NA | Reference | NA | Reference | NA | Reference | NA |
| Either | 1.48 (0.61-3.59) | 0.384 | 1.50 (0.60-3.75) | 0.383 | 1.55 (0.61-3.92) | 0.357 | 1.50 (0.62-3.60) | 0.368 | |
| Both | 8.36 (2.86-24.44) | <0.001 | 8.73 (2.86-26.62) | <0.001 | 8.56 (2.83-25.90) | <0.001 | 7.82 (2.80-21.87) | <0.001 | |
| PCI group | |||||||||
| qn-HRP and ql-HRP | None | Reference | NA | Reference | NA | Reference | NA | Reference | NA |
| Either | 0.66 (0.15-2.89) | 0.581 | 0.51 (0.11-2.28) | 0.380 | 0.79 (0.19-3.34) | 0.753 | 0.73 (0.16-3.38) | 0.683 | |
| Both | 0.81 (0.20-3.37) | 0.774 | 0.69 (0.16-3.05) | 0.624 | 1.08 (0.27-4.36) | 0.917 | 0.93 (0.25-3.48) | 0.909 | |
| This analysis was done in the whole population (n=697). *Model 1: adjusted for male, hyperlipidaemia, and acute coronary syndrome. †Model 2: adjusted for the use of aspirin, P2Y12 inhibitor, and statin. §Model 3: adjusted for LAD, % diameter stenosis, FFR, and LAP volume. CI: confidence interval; FFR: fractional flow reserve; HR: hazard ratio; HRP: high-risk plaque; LAD: left anterior descending artery; LAP: low-attenuation plaque; NA: not available; PCI: percutaneous coronary intervention; ql-HRP: qualitative HRP; qn-HRP: quantitative HRP; VOCO: vessel-oriented composite outcome | |||||||||
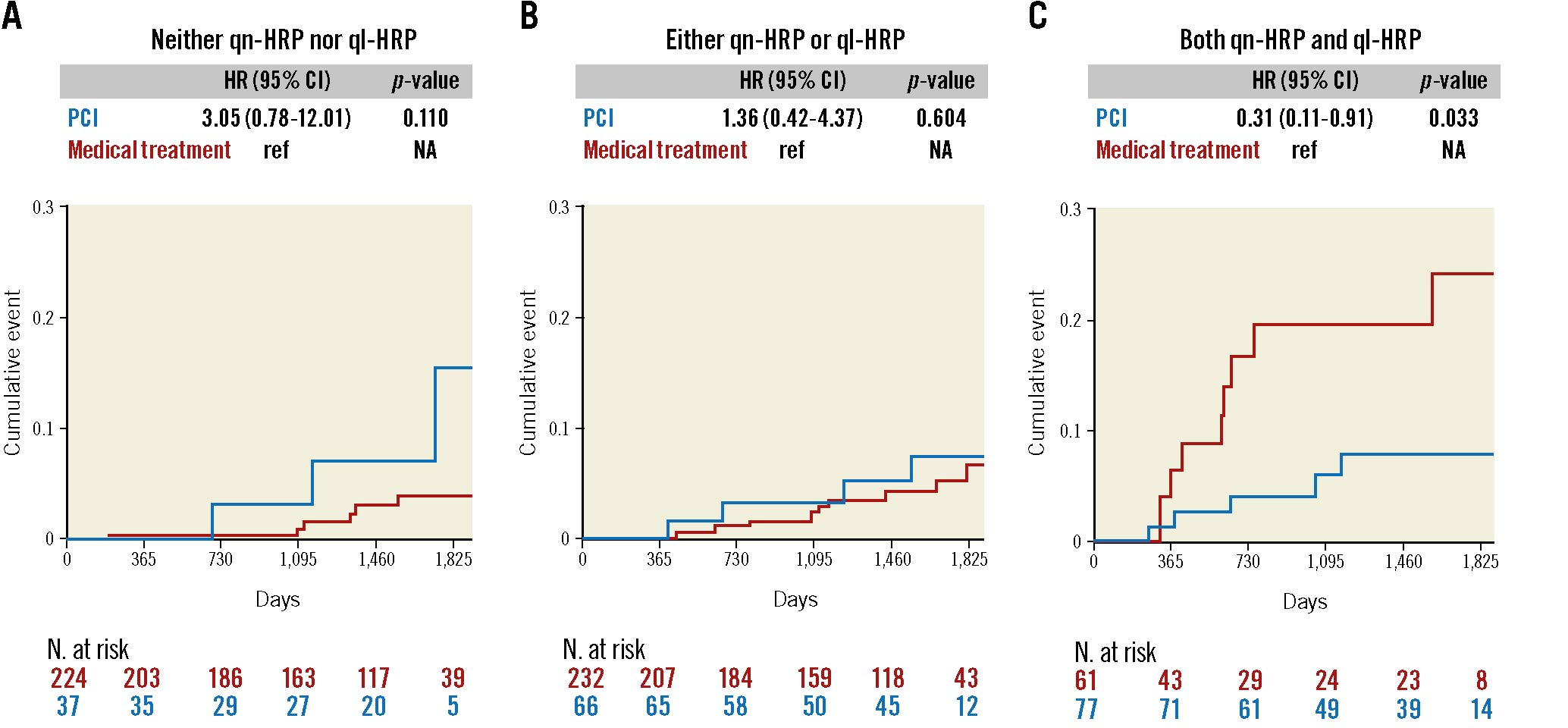
Figure 2. Outcome comparison between the medical treatment and PCI groups in lesion subsets divided by HRP. There was no difference in outcomes between the medical treatment and PCI groups in lesions with A) neither qn-HRP nor ql-HRP and B) either qn-hRP or ql-HRP, while C) the PCI group showed a better outcome in lesions with both qn-HRP and ql-HRP as compared with the medical treatment group. This analysis was done in the whole population (n=697). qn-HRP was defined as a lesion with an MLA <3.3 mm2 and a plaque burden ≥70.0%, and ql-HRP as with low-attenuation plaque or positive remodelling. CI: confidence interval; HR: hazard ratio; HRP: high-risk plaque; MLA: minimum lumen area; NA: not available; PCI: percutaneous coronary intervention; ql-HRP: qualitative HRP; qn-HRP: quantitative HRP
Table 3. The risk of VOCO in the PCI group relative to the medical treatment group among lesions with qn-HRP and ql-HRP.
| Unadjusted HR (95% CI)* | p-value | Model 1 HR (95% CI)* | p-value | Model 2 HR (95% CI)† | p-value | Model 3 HR (95% CI)§ | p-value | |
|---|---|---|---|---|---|---|---|---|
| Medical treatment | Reference | NA | Reference | NA | Reference | NA | Reference | NA |
| PCI | 0.31 (0.11-0.91) | 0.033 | 0.26 (0.06-1.05) | 0.058 | 0.23 (0.07-0.75) | 0.015 | 0.25 (0.07-0.97) | 0.044 |
| This analysis was done in lesions with both qn-HRP and ql-HRP (n=138). *Model 1: adjusted for male, hyperlipidaemia, and acute coronary syndrome. †Model 2: adjusted for the use of aspirin, P2Y12 inhibitor, and statin. §Model 3: adjusted for LAD, % diameter stenosis, FFR, and LAP volume. CI: confidence interval; FFR: fractional flow reserve; HR: hazard ratio; HRP: high-risk plaque; LAD: left anterior descending artery; LAP: low-attenuation plaque; NA: not available; PCI: percutaneous coronary intervention; ql-HRP: qualitative HRP; qn-HRP: quantitative HRP; VOCO: vessel-oriented composite outcome | ||||||||
Prognostic interaction between HRP and treatment type according to FFR
In the whole population, there was a significant prognostic interaction between PCI vs medical treatment and HRP (p for interaction=0.012) (Figure 3). When lesions were divided into the 2 FFR strata (FFR of 0.81–0.90 and>0.90), this interaction was observed in lesions with an FFR of 0.81–0.90, but not in those with an FFR>0.90 (Figure 3). In lesions with both qn-HRP and ql-HRP, the PCI group had better outcomes than the medical treatment group in the FFR stratum of 0.81-0.90 (HR 0.19, 95% CI: 0.04-0.90; p=0.036) but not in that of>0.90 (HR 0.61, 95% CI: 0.11-3.47; p=0.578). This result was consistent after adjusting for the clinical and lesion characteristics (Supplementary Table 3). Overall results were similar in the sensitivity analyses with lesion-oriented composite outcomes and patient-oriented composite outcomes (Supplementary Figure 4, Supplementary Figure 5).
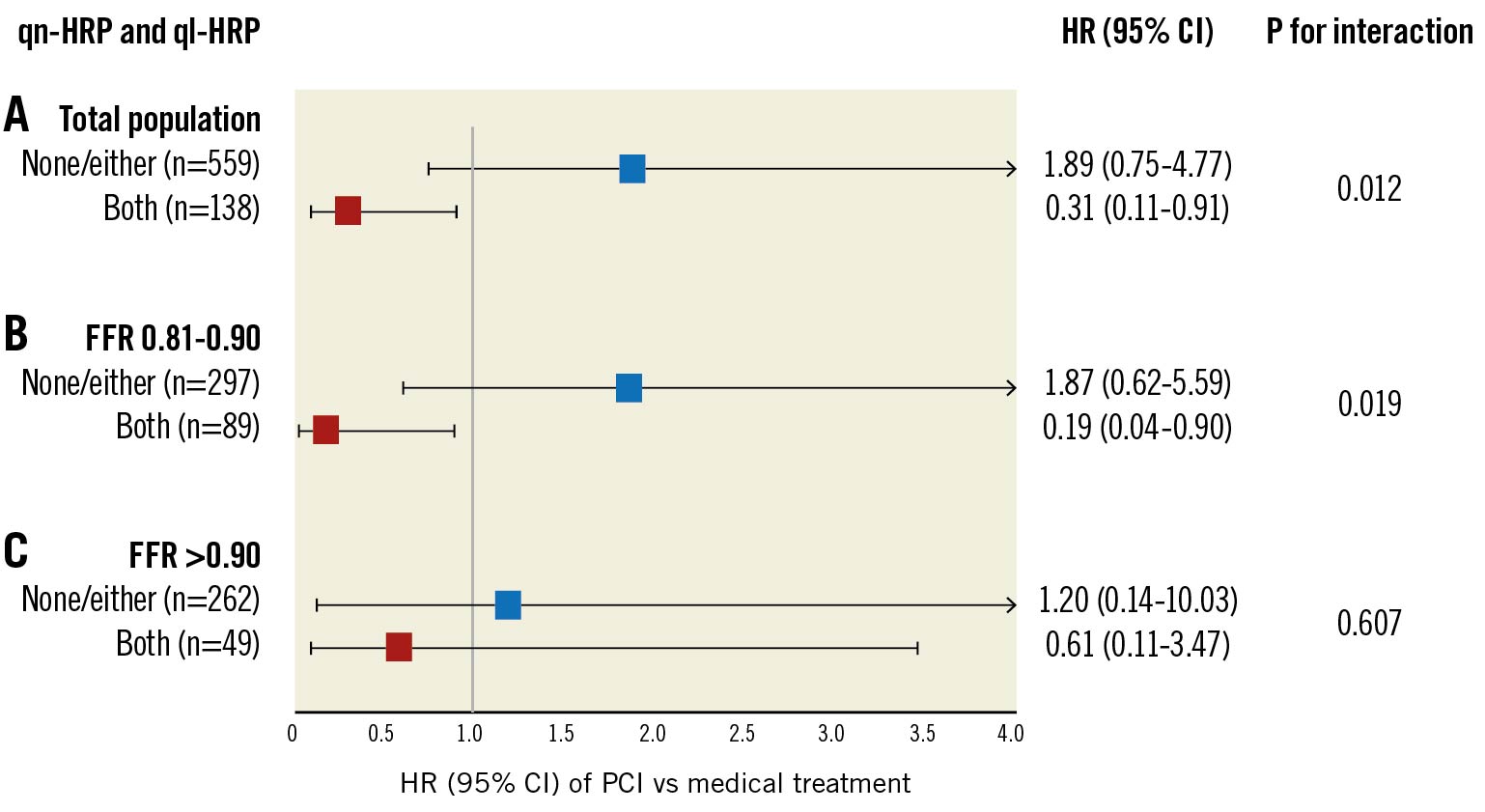
Figure 3. Prognostic interactions of HRP and treatment type according to FFR. A) There was a significant prognostic interaction between qn-HRP and ql-HRP with the treatment type in the total population. When lesions were stratified according to FFR, this prognostic interaction was predominantly observed B) in FFR of 0.81-0.90, C) but not in FFR >0.90. qn-HRP was defined as a lesion with an MLA <3.3 mm2 and a plaque burden ≥70.0%, and ql- HRP as with low-attenuation plaque or positive remodelling. CI: confidence interval; FFR: fractional flow reserve; HR: hazard ratio; HRP: high-risk plaque; MLA: minimum lumen area; PCI: percutaneous coronary intervention; ql-HRP: qualitative HRP; qn-HRP: quantitative HRP
Discussion
The current study investigated the prognostic role of individual and combined quantitative and qualitative plaque measures in non-ischaemic lesions and their interactions with treatment modality and physiologic lesion severity. The main findings of the study were as follows: 1) CCTA-derived qn-HRP or ql-HRP was associated with an increased risk of VOCO among non-ischaemic lesions. When lesions were divided into qn-HRP and ql-HRP, only lesions with both were associated with an increased risk of VOCO. 2) Compared to the medical treatment group, the PCI group had a better outcome in lesions with both qn-HRP and ql-HRP, but not in lesions with none or either of them. 3) A significant prognostic interaction between HRP and treatment strategy was found in the FFR range of 0.81–0.90, but not in that of>0.90.
Prognostic value of quantitative and qualitative plaque characteristics
HRP contains quantitative and qualitative plaque features associated with a risk of coronary events and can be evaluated using various imaging modalities67891011. Recent studies have shown this association, even in non-ischaemic lesions. The presence of adverse characteristics in CCTA or intravascular ultrasound (IVUS) was associated with a 3-fold increased risk of VOCO in vessels with an FFR>0.801213. In the COMBINE OCT-FFR study, the detection of thin-cap fibroatheroma on optical coherence tomography (OCT) was related to a 5-fold higher rate of adverse events among FFR-negative lesions in diabetic patients15. However, most previous studies did not discriminate plaque quantity and quality components when defining HRP. From the perspective of clinical applicability, an investigation on whether there are replaceable or mutual implications of both components is needed because IVUS is generally considered better suited to define quantitative plaque measures with its penetration ability while OCT is better suited to lumen assessment and qualitative plaque measures with its excellent resolution; CCTA is suitable for non-invasively assessing both aspects in whole vessels despite its lower resolution than invasive imaging. In the current study, we defined qn-HRP and ql-HRP as the best plaque metrics among possible combinations of quantitative and qualitative plaque characteristics to avoid a biased definition for 1 side and found that qn-HRP and ql-HRP were associated with worse outcomes, but only lesions with both qn-HRP and ql-HRP showed the increased risk of VOCO. Moreover, there was an additive outcome discrimination ability between the 2. This finding is in line with the results of the PROSPECT II study, which showed the highest event rate in lesions with a plaque burden ≥70% and higher maximum lipid core burden index11, and the study by Lee et al, which reported the additive value of plaque burden and high-risk plaque in the prediction of rapid lesion progression and adverse outcomes16.
Outcomes with medical treatment and PCI in lesions with HRP
Although a variety of plaque characteristics are prognostic markers of CAD, there is a paucity of data on their role as an indicator for revascularisation. In a pilot randomised controlled study with non-ischaemic lesions with a plaque burden ≥65%, bioresorbable vascular scaffold-treated lesions had a higher MLA and lower rate of clinical events at 2 years than medically treated lesions, suggesting the safety and potential effectiveness of PCI for those lesions24. Thus, the clinical question about the benefit of PCI for high-risk lesions not causing myocardial ischaemia has been raised. To address this issue in the current study, we compared the outcomes of medically treated non-ischaemic lesions (FFR>0.80) with those of revascularised lesions that achieved non-ischaemic status after PCI (post-PCI FFR>0.80) according to high-risk plaque status. As a result, the rate of VOCO serially increased in the order of neither, either, and both qn-HRP and ql-HRP in the medical treatment group, but there was no such trend in the PCI group. Moreover, when we compared the clinical outcomes between the medical treatment and PCI groups in each category, the PCI group showed better clinical outcomes than the medical treatment group in lesions with both qn-HRP and ql-HRP, whereas the 2 treatment strategies did not have different outcomes in lesions with either or none of them. Our findings align with the plaque-sealing effect of stent implantation2526, the benefit of which might exceed the risk of PCI-associated events in high-risk non-ischaemic lesions27, which can only be identified by assessing both plaque quantity and quality.
Interaction between treatment modalities and HRP according to FFR strata and its clinical implications
The numerical value of FFR provides a risk continuum for clinical events throughout both ischaemic and non-ischaemic ranges28 and is also intertwined with plaque burden and characteristics1429. To examine the practical implications of the numeric FFR value in lesions with an FFR>0.80 in consideration of plaque characteristics, we tested the prognostic interaction between PCI and both the presence of qn-HRP and ql-HRP in lesions with an FFR of 0.81-0.90 and>0.90. In the presence of qn-HRP and ql-HRP, the PCI group still showed a better prognosis than the medical treatment group in lesions with an FFR of 0.81-0.90, but this association was attenuated in lesions with an FFR>0.90. This finding is due to very low clinical events in lesions with an FFR>0.9030 and suggests that FFR strata should be considered in the risk assessment of non-ischaemic lesions in addition to plaque characteristics. Based on these observations, a scheme for the risk assessment of FFR-negative lesions can be proposed (Central illustration). In lesions with an FFR>0.80, those with an FFR>0.90 can be safely deferred without further diagnostic testing. Plaque quantity assessment may be needed for lesions with an FFR strata of 0.81–0.90, and low risk can be expected in those without qn-HRP. If qn-HRP is present, additional assessment of plaque quality is warranted, and those without ql-HRP can also be safely deferred. Lesions with both qn-HRP and ql-HRP should be regarded as high-risk lesions that might require meticulous medical treatment or revascularisation.
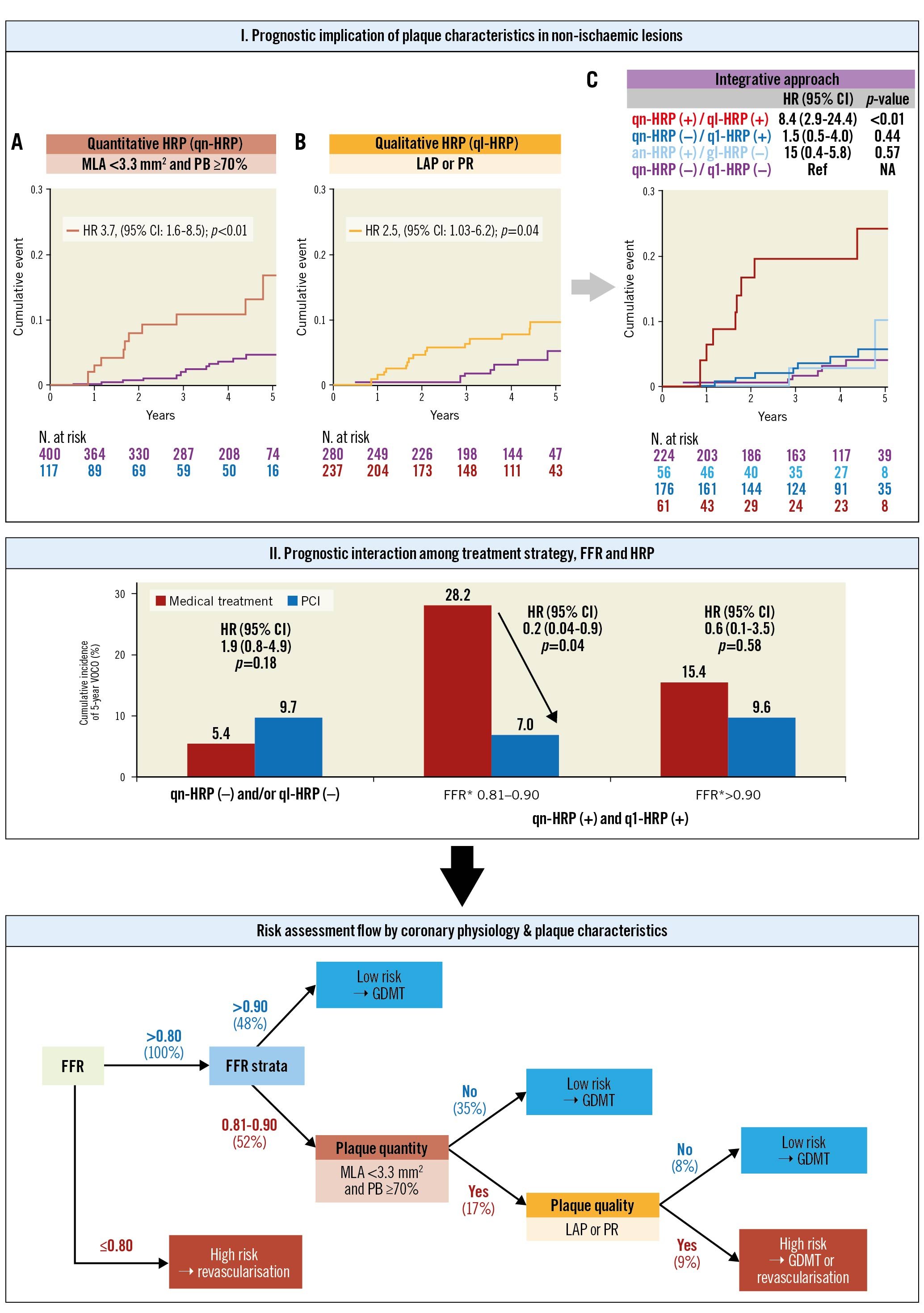
Central illustration. Risk assessment strategy of non-ischaemic lesions according to physiologic lesion severity and quantitative and qualitative plaque characteristics. The presence of qn-HRP or ql-HRP was associated with an increased risk of VOCO, but only the lesions with both qn-HRP and ql-HRP showed a higher risk than the lesions without them. When lesions were stratified by FFR strata, qn-HRP, and ql-HRP, the PCI group showed a lower risk for VOCO than the medical treatment group in lesions with both qn-HRP and ql-HRP and FFR of 0.81–0.90. Based on these observations, a risk assessment strategy for non-ischaemic lesions was proposed. qn-HRP: MLA <3.3 mm2 and plaque burden ≥70.0%, ql-HRP: low-attenuation plaque or positive remodelling. *In the PCI group, revascularisation was performed in low pre-PCI FFR (≤0.80) lesions, and post-PCI FFR was designated as the FFR value of the corresponding vessel. CI: confidence interval; FFR: fractional flow reserve; GDMT: guideline-directed medical treatment; HR: hazard ratio; HRP: high-risk plaque; LAP: low-attenuation plaque; MLA: minimum lumen area; PB: plaque burden; PCI: percutaneous coronary intervention; PR: positive remodelling; ql-HRP: qualitative HRP; qn-HRP: quantitative HRP; VOCO: vessel-oriented composite outcomes
Limitations
Several limitations of the current study should be noted. This is a post hoc analysis of a pooled registry and warrants future studies to confirm the current findings in a prospective manner. The PCI group included lesions with a low pre-PCI FFR, which may indirectly represent the outcomes after PCI for non-ischaemic lesions. To minimise potential bias, only revascularised lesions that achieved a post-PCI FFR>0.80 were included in the PCI group in our study. The number of cases is relatively small, so the results should be interpreted as only hypothesis-generating. Due to a relatively low rate of clinical events in the current registry, future studies with a larger number of patients and events are needed to generalise our findings. Clinical endpoints included revascularisation events, and the hard outcome could not be analysed separately because of the small number of events. Invasive imaging was not included. Given that the spatial resolution of the CCTA is lower than that of intravascular imaging such as IVUS or OCT, intravascular imaging-based plaque analysis may reveal different findings. Therefore, our results may need to be validated with plaque features derived from other imaging modalities. Since the current study population consisted of patients with mixed clinical presentations, stable CAD, and acute coronary syndrome, the results may not be generalisable across all CAD categories. Local haemodynamic parameters such as shear stress were not interrogated in the current results, and the prognostic implications of them, along with our findings, need to be investigated in future studies.
Conclusions
In non-ischaemic lesions, ql-HRP and qn-HRP showed an incremental value in risk assessment and had prognostic interactions with FFR strata and treatment types. An integrative assessment of physiological lesion severity and quantitative and qualitative plaque characteristics is needed for risk stratification and selection of appropriate treatment strategies.
Impact on daily practice
In the prediction of clinical outcomes in lesions without myocardial ischaemia, quantitative high-risk plaque (qn-HRP) was defined as an MLA<3.3 mm2 and a plaque burden ≥70.0%, and qualitative high-risk plaque (ql-HRP) as low-attenuation plaque or positive remodelling. qn-HRP and ql-HRP showed a synergistic prognostic impact on the clinical outcomes. In lesions with both qn-HRP and ql-HRP, the PCI group showed a better prognosis than the medical treatment group, and this association was consistently observed in those with an FFR of 0.81-0.90.
Conflict of interest statement
B-K. Koo received institutional research grants from Abbott Vascular and Philips Volcano. J.H. Doh received research grants from Philips Volcano. The other authors have no conflicts of interest to declare relevant to the submitted work.
Supplementary data
To read the full content of this article, please download the PDF.

