Abstract
Aims: Contrast-flow quantitative flow ratio (cQFR) is a novel index of the functional severity of coronary stenosis, which can be calculated from three-dimensional quantitative coronary angiography. Previous studies have shown a high correlation between cQFR and fractional flow reserve. This study sought to investigate the prognostic value of the sum of cQFR in three vessels (3V-cQFR) in patients with stable coronary artery disease (CAD).
Methods and results: A total of 549 patients who underwent invasive coronary angiography and cQFR measurements in three vessels were analysed in the present study. Median cQFR of all cQFR-assessed vessels and 3V-cQFR of each patient were 0.94 (0.85-0.98) and 2.75 (2.62-2.87), respectively. During a median follow-up of 2.2 years, 57 patients experienced MACE. 3V-cQFR could provide prognostic information in the total cohort and among those without undergoing revascularisation as well. In a multivariate analysis, 3V-cQFR, high-sensitivity cardiac troponin-I and previous MI remained as independent predictors for MACE, and conventional angiographic scores did not.
Conclusions: 3V-cQFR could discriminate the risk for MACE in patients with stable CAD. 3V-cQFR calculated from routine invasive angiograms was feasible, and the prognostic implication could be more powerful than that of conventional angiographic scores.
Introduction
Fractional flow reserve (FFR) is regarded as a standard method to evaluate functional ischaemia in epicardial coronary artery disease (CAD). While FFR is routinely used for guiding revascularisation, FFR also holds a prognostic efficacy in a continuous manner1. A recent study has shown the prognostic value of the total sum of FFR in three vessels, based on the assumption that the assessment could implicate the total atherosclerotic burden2. However, FFR measurement is invasive, costly and time-consuming, and the induction of hyperaemia sometimes causes discomfort. For these reasons, routine assessment of three-vessel FFR might not be practical.
Quantitative flow ratio (QFR) is a novel method for evaluating the functional significance of epicardial stenosis on the basis of three-dimensional quantitative angiography (3D-QCA) and fluid dynamics algorithms3. The diagnostic accuracy of contrast-flow QFR (cQFR) in identifying functionally significant coronary stenosis was confirmed in several recent studies3,4. Since cQFR can be calculated from routine angiographic images, cQFR may potentially elaborate the angiography-based assessment of functional ischaemia.
In this study, we aimed to investigate the prognostic implication of total physiological atherosclerotic burden assessed by summing cQFR in three vessels.
Methods
PATIENT POPULATION
Consecutive patients who presented with known or suspected CAD and who underwent elective coronary angiography (CAG) at Tsuchiura Kyodo General Hospital (Tsuchiura, Ibaraki, Japan) from September 2014 to September 2016 were retrospectively investigated. The study flow chart is shown in Figure 1. The study protocol excluded patients who were referred for CAG not primarily for CAD assessment, patients with previous coronary artery bypass surgery, renal insufficiency with baseline creatinine >1.5 mg/dl, presence of unstable symptoms (worsening angina or rest angina within one month), and myocardial infarction (MI) episode or cardiac catheterisation within three months before the CAG. For QFR analysis, atrial fibrillation (AF) or frequent premature beat during CAG, ostial lesions <3 mm from the aorta, severe vessel overlap or tortuosity at the stenotic segments, luminal reduction caused by myocardial bridge, poor angiographic image quality in which optimal 3D-QCA models could not be constructed, and side branch lesions were excluded. In order to conduct QCA analysis in three vessels, patients with the presence of totally occluded vessels were also excluded. QFR analyses were performed only for main branches. In patients with small vessels (proximal reference diameter <2 mm) of the right coronary artery (RCA) or the left circumflex coronary artery (LCx), QFR analyses were performed for the remaining two vessels omitting the small artery. There were no patients with two small main branches in the present cohort. The study was conducted in accordance with the Declaration of Helsinki. The institutional ethics committee approved the study protocol and all patients provided written informed consent for enrolment in the institutional database for potential future investigations. All patient data and procedural details were obtained from medical records.
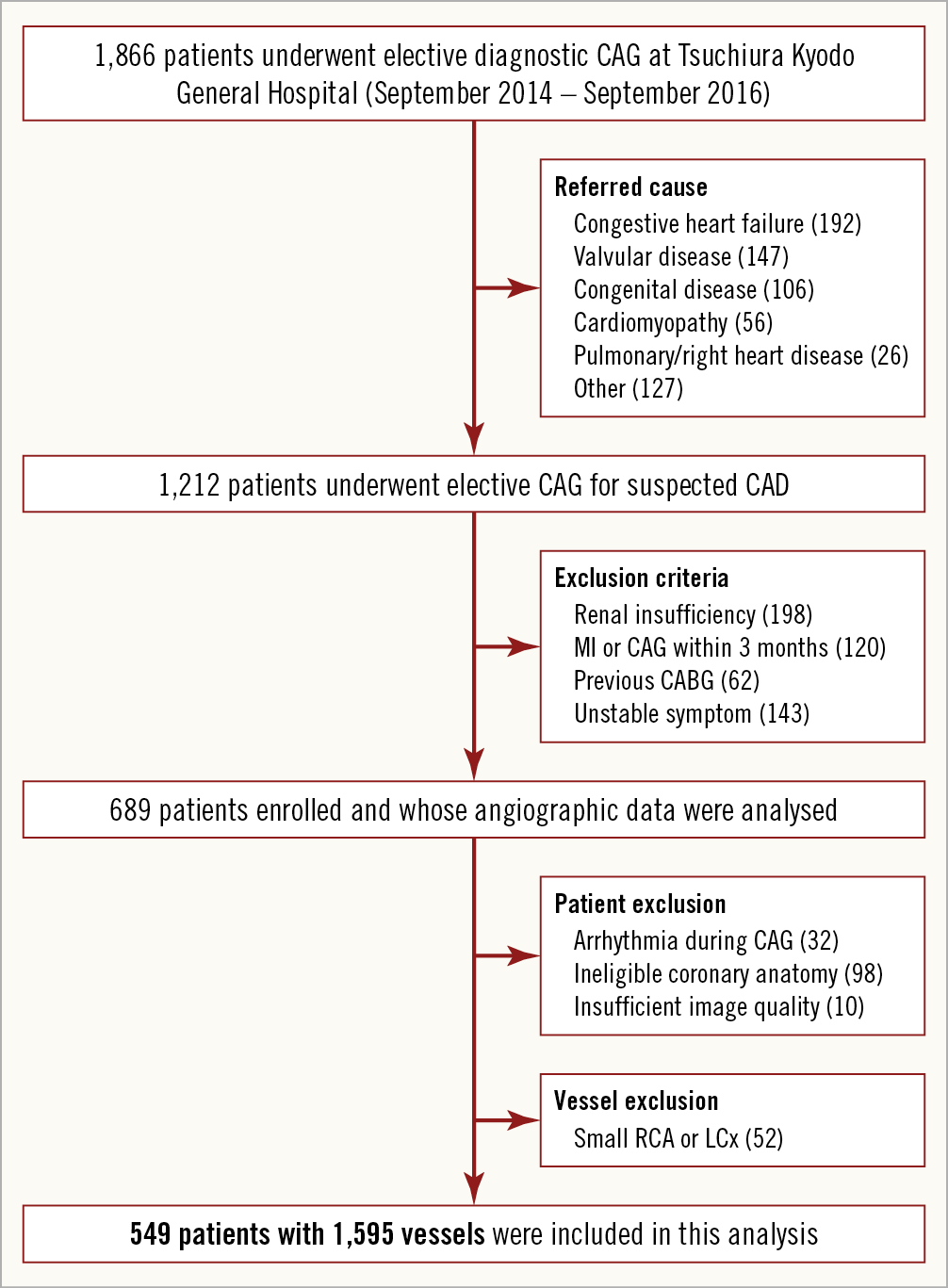
Figure 1. Study flow chart.
BIOCHEMICAL MEASUREMENT ANALYSIS
All the enrolled patients underwent baseline high-sensitivity cardiac troponin-I (hs-cTnI) measurement from blood samples obtained before CAG, using the ARCHITECT i2000SR STAT hs-cTnI assay (Abbott Laboratories, North Chicago, IL, USA). Sampling was conducted in the morning in clinically stable patients in a fasting state.
QUANTITATIVE ANALYSES ON ANGIOGRAMS
The complexity of coronary lesions was assessed by SYNTAX score and Gensini score5. SYNTAX score was defined using the online, most recently updated calculator (SYNTAX score I from http://www.SYNTAXscore.com). The Gensini score was calculated as equal to the sum of all segment scores, which were determined as segment weighting factor multiplied by a severity score. Segment weighting factors were between 0.5 and 5.0 depending on the arterial segments. Severity scores reflecting the specific percent diameter stenosis of the coronary artery segment were 32, 16, 8, 4, 2, and 1, for 100%, 99%, 90%, 75%, 50%, and 25% stenosis, respectively5. Multivessel disease was defined as the presence of ≥2 vessels with 3D-QCA diameter stenosis >50%.
QFR COMPUTATION AND THREE-VESSEL cQFR
The 3D-QCA analysis and QFR computation were performed using a validated software (QAngio XA 3D 1.1.0; Medis medical imaging systems, Leiden, the Netherlands) by two independent investigators (M. Hoshino and Y. Kanno) who were blinded to the patient information and were well trained before this analysis6. Two angiographic projections acquired after nitroglycerine administration at different angles ≥30° apart were transferred by local network to the QFR system. From two end-diastolic frames, the investigator identified one to two anatomical landmarks as reference points for matching location information, and vessel contours were automatically delineated. Based on the reconstructed 3D anatomical vessel model, 3D-QCA analyses and fixed-flow QFR (fQFR) were determined. Then, the contrast flow model was applied, in which cQFR was computed by the contrast flow velocity based on the Thrombolysis In Myocardial Infarction (TIMI) frame count analysis6. All the CAG images were acquired with a frame rate of 30/sec.
Three-vessel cQFR (3V-cQFR) was calculated as the sum values of cQFR in three vessels. In 52 cases with small RCA or LCx, 3V-cQFR was calculated as a mean value of cQFR in the other two vessels multiplied by three, according to three-vessel FFR study2. The 3V-cQFR was based on the index CAG results, before modification by early revascularisation. A representative example of the 3D-QCA analyses and QFR computation is shown in Figure 2.
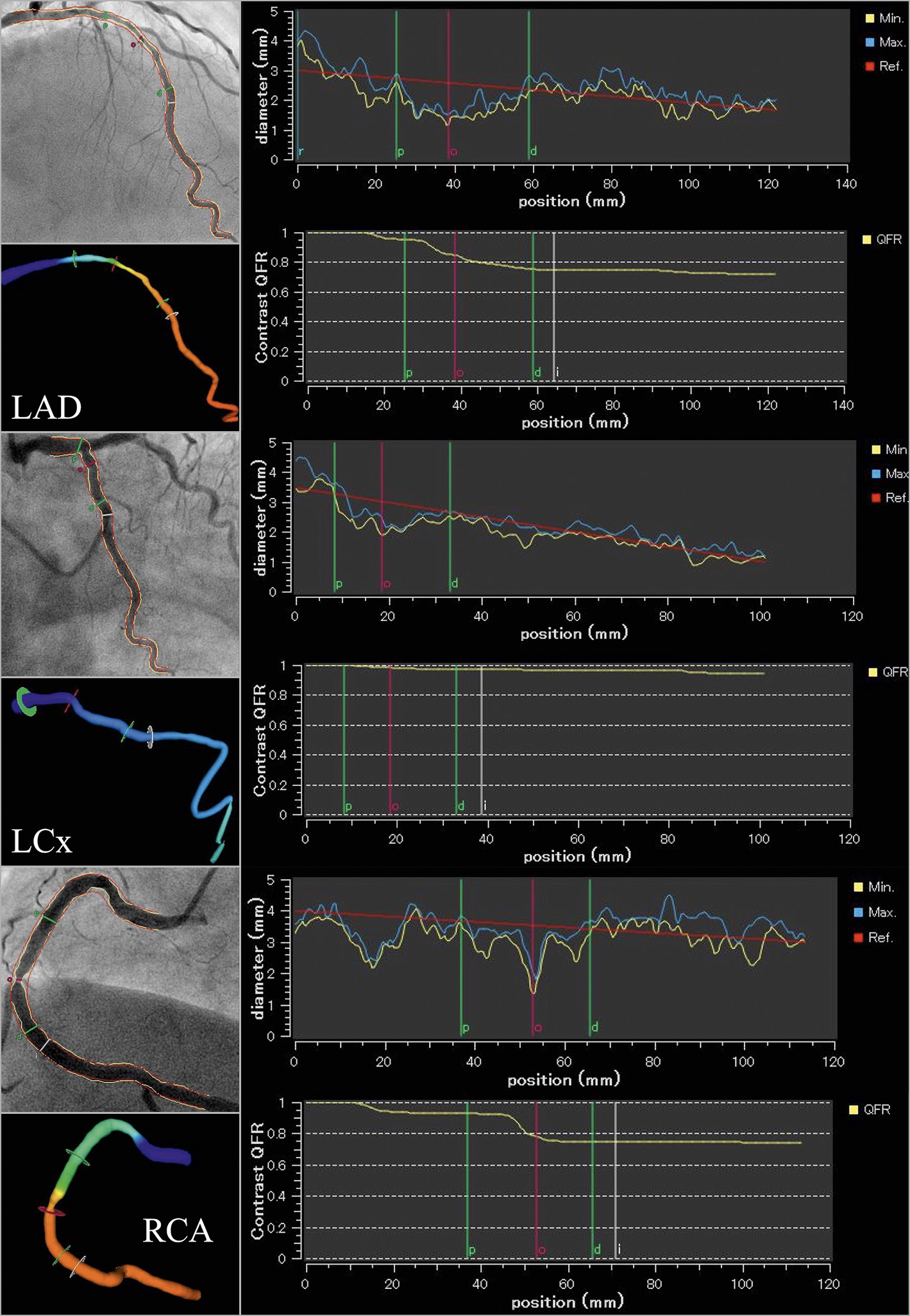
Figure 2. Representative cQFR analysis. A representative case undergoing three-vessel quantitative flow ratio (QFR) measurement. In each vessel, vessel contours were delineated from two different views of coronary angiograms (left upper panel), the information on Thrombolysis In Myocardial Infarction frame count was added (left lower panel), and contrast-flow QFR (cQFR) was determined (right panels). The cQFR values of diffuse disease in LAD, non-significant stenosis in LCx, and focal stenosis in RCA were 0.72, 0.95, and 0.74, respectively. Thus, the three-vessel cQFR (3V-cQFR) was 0.72+0.95+0.74=2.41.
FFR MEASUREMENT
A total of 154 vessels had undergone FFR measurement with a Certus™ coronary pressure wire (St. Jude [now Abbott], St. Paul, MN, USA) in the present cohort. The agreement between FFR and cQFR or fQFR was tested in this subgroup. FFR was calculated as the ratio of distal coronary pressure to proximal coronary pressure at stable hyperaemia induced by intravenous adenosine infusion (140 μg/kg/min).
INDICATION FOR REVASCULARISATION AND CLINICAL FOLLOW-UP
Clinical follow-up data were collected via a review of the medical records and/or telephone interviews. Major adverse cardiac events (MACE) were defined as a composite of all-cause death, non-fatal MI, and clinically driven revascularisation. MI was diagnosed based on the third universal definition of MI. Clinically driven revascularisation (remote revascularisation) was applied to ischaemia-driven revascularisation of any vessels based on a positive non-invasive test or physiologic test at least three months after the index CAG. All revascularisations required or scheduled based on the index CAG results (defined as early revascularisation) were performed within three months after the procedures.
STATISTICAL ANALYSIS
Categorical data, expressed as frequencies and percentages, were compared using the χ2 test or Fisher’s exact test, as appropriate. Continuous biochemical or physiological data were expressed as median (interquartile range [IQR]) or mean±standard deviation (SD) and analysed using the Mann-Whitney test or Kruskal-Wallis test for variables with non-normal distribution or using the Student’s t-test for those with normal distribution. Event rates over time were estimated using the Kaplan-Meier method, and linear trends were tested with log-rank tests. A Cox proportional hazards regression model was used to calculate hazard ratio (HR) and 95% confidence interval (CI) and to identify predictors of MACE. The covariates used in multivariate analysis were selected with the criterion of p<0.01 in the univariate analysis, which included diabetes mellitus, previous MI, hs-cTnI, multivessel disease, Gensini score and 3V-cQFR. The predictive power of cQFR, fQFR or diameter stenosis for remote revascularisation was assessed by receiver operating characteristic (ROC) curve analysis and the areas under the curve (AUC) were compared. All statistical analyses were performed using JMP 11.2.0 (SAS Institute Inc., Cary, NC, USA). A two-sided p<0.05 was considered statistically significant.
Results
BASELINE CLINICAL FEATURES
From 689 patients enrolled in the present study, 32 with AF or frequent premature beat, 22 with severe tortuosity or vessel overlap, 48 with the presence of totally occluded vessels, 11 with side branch lesions, 17 with ostial lesions, and 10 with poor angiographic image quality were excluded. In addition, 52 small vessels were excluded. Therefore, a total of 549 patients (79.7%) with 1,595 vessels were included in the present analysis (Figure 1).
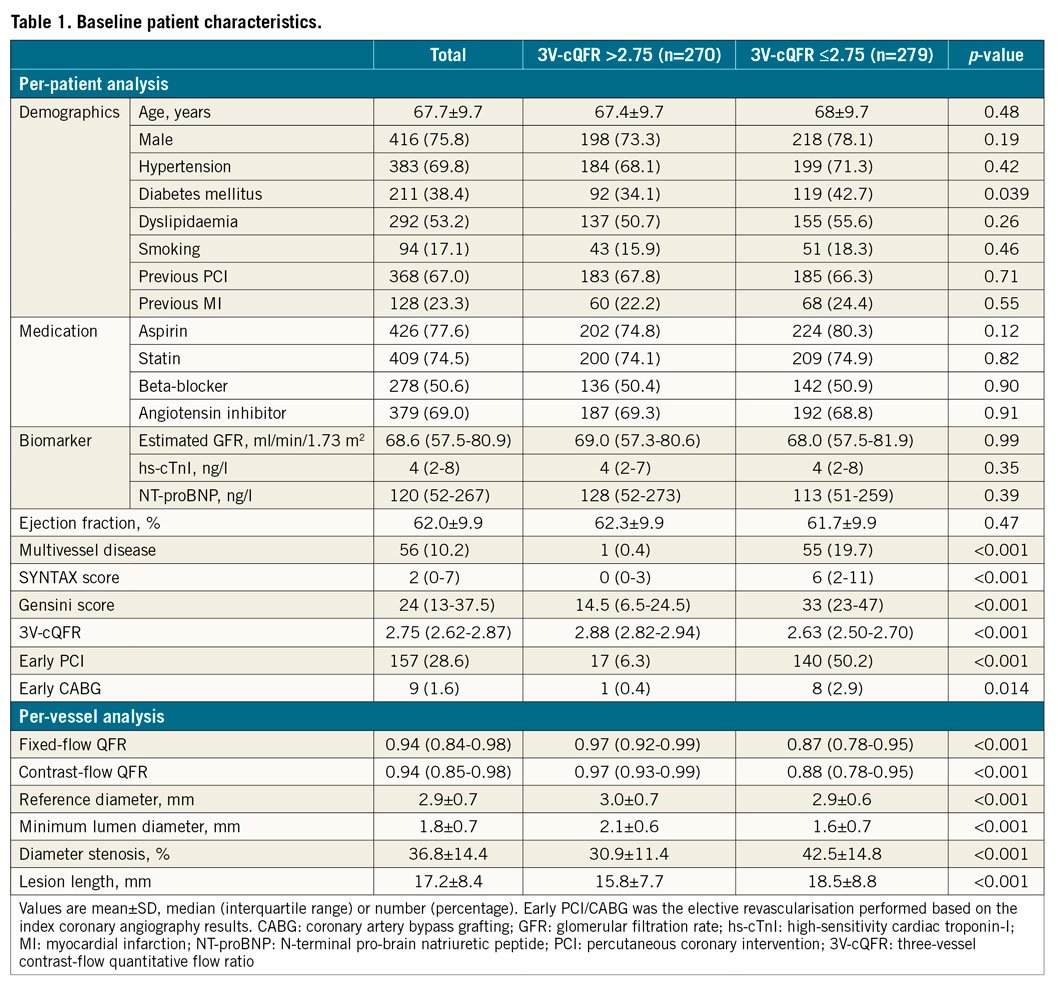
In the total cohort, median cQFR of all cQFR-assessed vessels and 3V-cQFR were 0.94 (0.85-0.98) and 2.75 (2.62-2.87), respectively. The number of completely normal vessels, defined as cQFR=1.0, was 158 out of 1,595 vessels. There were significant correlations between 3V-cQFR and SYNTAX score (R=−0.57, p<0.001) or Gensini score (R=−0.60, p<0.001). The clinical characteristics and angiographic data in patients divided according to the median 3V-cQFR are summarised in Table 1.
CLINICAL OUTCOMES
The median follow-up period was 2.2 (1.7-2.7) years, during which six patients experienced non-fatal MI, 10 patients died, and 41 patients needed ischaemia-driven revascularisation (overall MACE rate: 57 of 549 [10.4%]). Patients with MACE had lower cQFR in all three vessels than those without MACE (all: p<0.05), resulting in lower 3V-cQFR than in patients without MACE (2.76 [2.64-2.88] vs. 2.64 [2.49-2.73], p<0.001) (Table 2). hs-cTnI levels were significantly higher in patients with MACE compared to those without MACE (83,4,5,6,7,8,9,10,11,12,13 vs. 42,3,4,5,6,7, p<0.001). Figure 3 presents Kaplan-Meier curves showing survival from MACE in patients divided according to median 3V-cQFR. MACE incidence was significantly higher in patients with lower 3V-cQFR (p<0.001).
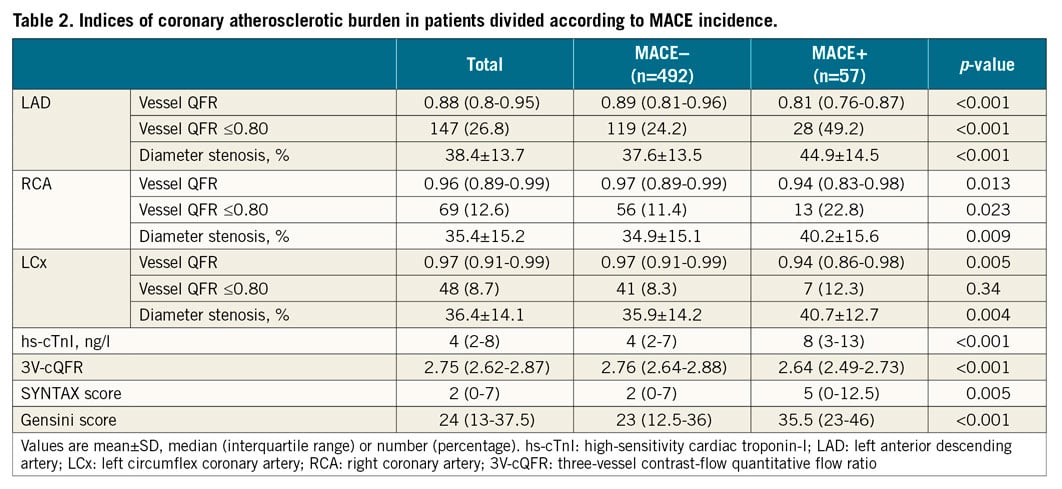
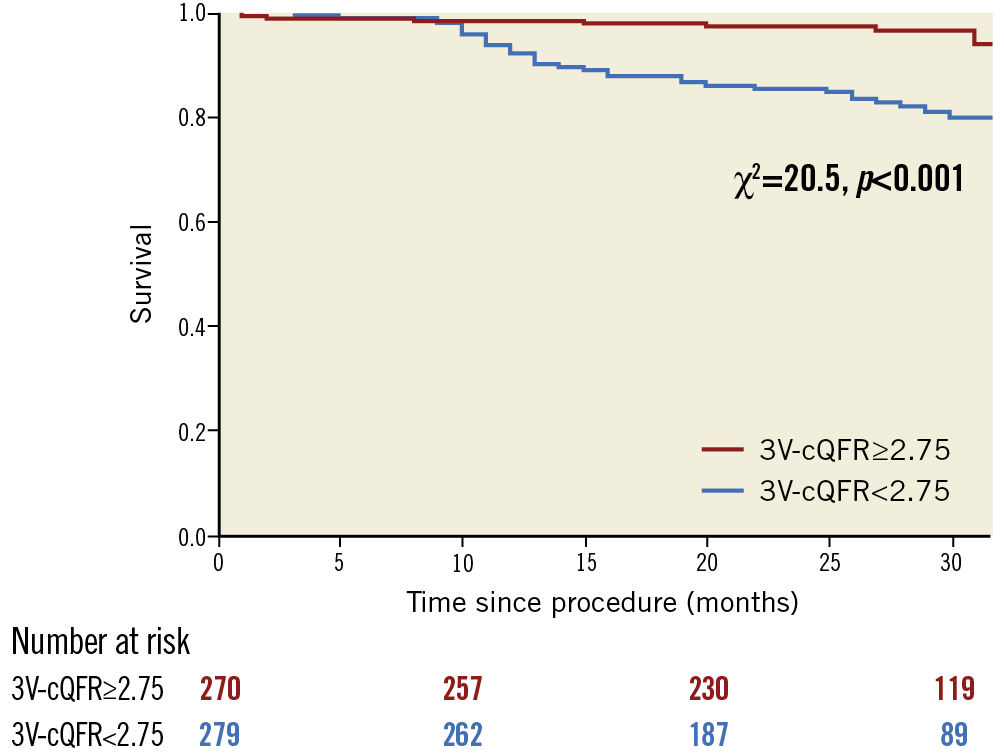
Figure 3. Survival from MACE in patients divided by the median value of 3V-cQFR. Kaplan-Meier curves demonstrating the survival from major adverse cardiac events (MACE) in patients divided according to the median value of 3V-cQFR (2.75). 3V-cQFR significantly discriminated the incidence of MACE (log-rank p<0.001).
Univariate Cox proportional regression analyses revealed that male sex, presence of diabetes mellitus, previous MI, high hs-cTnI, low ejection fraction (EF), multivessel disease, high SYNTAX score, high Gensini score, and low 3V-cQFR were significantly associated with the incidence of MACE (Table 3). In a multivariate model, previous MI (HR 1.887, 95% CI: 1.068-3.278, p=0.029), high hs-cTnI (HR 1.481 per log-transformed ng/l, 95% CI: 1.133-1.909, p=0.005), and low 3V-cQFR (HR 0.971 per 0.01 unit, 95% CI: 0.955-0.988, p<0.001) were the independent predictors for MACE. The result indicated the prognostic implication of 3V-cQFR as a continuous index.
PROGNOSTIC EFFICACY OF 3V-cQFR IN PATIENTS WHO DID NOT UNDERGO EARLY REVASCULARISATION
A total of 392 patients (71.4%) had not undergone early revascularisation based on the index CAG results. In this subgroup, median values of 3V-cQFR were 2.81 (2.72-2.91), and 26 patients (6.6%) experienced MACE. The patients with 3V-cQFR <2.75 had a significantly increased incidence of MACE (p=0.022) (Figure 4). In the univariate Cox regression analyses, male sex, previous MI, hs-cTnI and 3V-cQFR were significantly associated with MACE incidence (Supplementary Table 1). SYNTAX score or Gensini score was not significantly related to MACE (p=0.45 and 0.50, respectively).
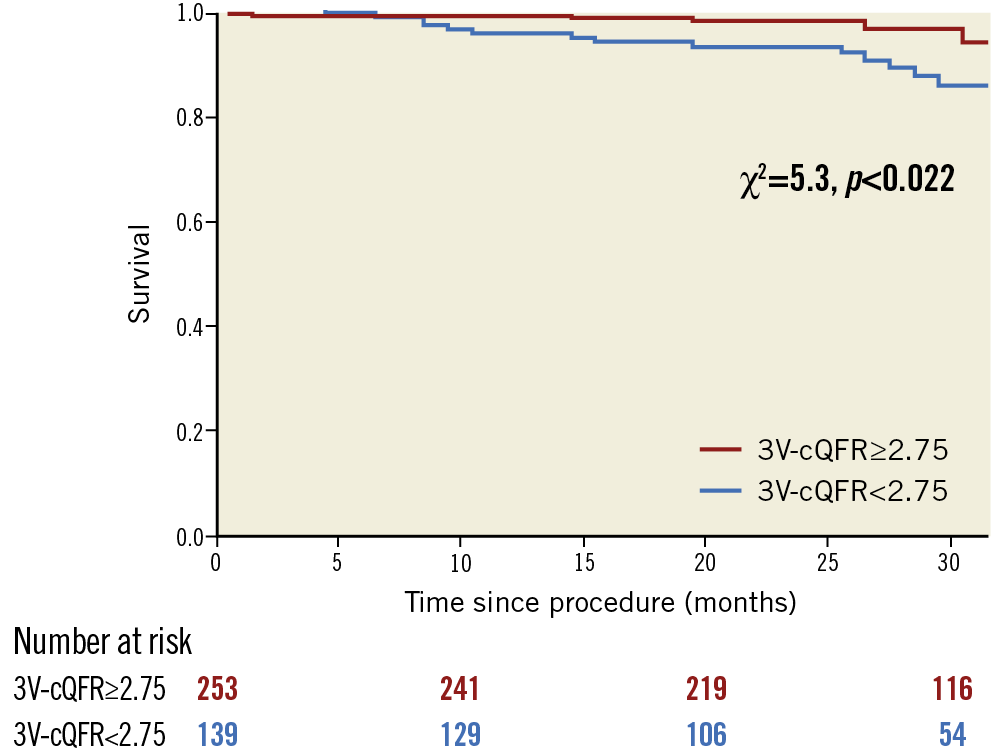
Figure 4. Survival from MACE in patients who had not undergone early revascularisation. Kaplan-Meier curves demonstrating the survival from MACE in patients who had not undergone early revascularisation divided according to 3V-cQFR of 2.75. 3V-cQFR significantly discriminated the incidence of MACE (log-rank p=0.022).
PER-VESSEL ANALYSIS ON cQFR AND CLINICAL OUTCOMES
Among 1,595 vessels, a total of 49 vessels needed remote revascularisation. Vessels requiring remote revascularisation had lower cQFR than those vessels not needing remote revascularisation (median 0.83 [0.76-0.91] vs. 0.94 [0.86-0.98], p<0.001). Supplementary Figure 1 summarises the incidence of remote revascularisation in vessels divided according to the combination of cQFR and early revascularisation. Revascularised vessels with cQFR ≤0.80 had the highest incidence of remote revascularisation (13/153, 8.5%), which was comparable to those with cQFR ≤0.80 which had not undergone early revascularisation (8/110, 7.3%, p=0.72).
cQFR could predict remote revascularisation more robustly than diameter stenosis (AUC: 0.73 [95% CI: 0.65-0.79], p<0.001 for cQFR; AUC: 0.66 [95% CI: 0.56-0.74], p<0.001 for diameter stenosis; p=0.043 for pairwise comparisons) (Supplementary Figure 2). The prognostic information was similar between fQFR and cQFR (AUC: 0.71 [95% CI: 0.61-0.79], p<0.001 for fQFR; p=0.29 for pairwise comparisons with cQFR).
AGREEMENT BETWEEN FFR AND cQFR
In 154 vessels in which FFR was measured, a good correlation was observed between FFR and cQFR (R=0.84, p<0.001) (Supplementary Figure 3). There was an acceptable agreement between these indices (mean difference: 0.014±0.062, p=0.007). The agreement was better than that between FFR and fQFR (mean difference: 0.032±0.072, p=0.001).
Discussion
This is the first study investigating the prognostic efficacy of 3V-cQFR in patients with stable CAD. The present study provides the following novel findings: 1) 3V-cQFR provided independent prognostic information for the occurrence of future adverse events in a continuous manner; 2) 3V-cQFR could provide more accurate risk stratification compared with established angiographic scores; 3) 3V-cQFR could provide prognostic information in patients who had not undergone early revascularisation; and 4) vessels with cQFR ≤0.80 which were not indicated for revascularisation had comparable prognosis to revascularised vessels with cQFR ≤0.80.
Recent studies have consistently shown the importance of understanding the high risk for future cardiovascular events in the “vulnerable” patient with stable symptoms7,8,9. The assessment of total coronary atherosclerotic burden would be closely linked to the identification of “vulnerable” patients. The PROSPECT trial has shown the association between total plaque burden quantified by intravascular ultrasound and adverse events in non-culprit vessels7. Computed tomography angiography-derived total plaque volume was higher in patients who had acute coronary syndrome compared with those without8. These studies have provided insights into total “anatomical” atherosclerotic burden; we have proposed 3V-cQFR as the index of total “physiologic” atherosclerotic burden. The present results imply the better utility of 3V-cQFR compared to traditional angiographic scores although these could be obtained by a pure angiogram. The prognostic implication of cQFR was in line with the recent study in patients with ST-elevation MI10.
Emphasis on the evaluation of total atherosclerotic burden relies on the evidence that non-culprit vessels can contribute to future cardiovascular events, which was directly confirmed in the PROSPECT study11. The 3V FFR-FRIENDS trial has investigated the prognostic efficacy of 3V-FFR relying on such evidence. In accordance with this study, we validated the prognostic efficacy of 3V-cQFR in the prediction of MACE in patients with stable CAD. As FFR is an invasive method to quantify epicardial coronary stenosis physiologically using drug-induced vasodilation, the procedure is invasive, time-consuming, costly, and can cause discomfort. Calculation of cQFR is based on TIMI frame counting without pressure wire or inducing hyperaemia, and only needs two different angiograms3,4. Although cQFR assessment has several limitations and about 20% of cases could not undergo 3V-cQFR analyses in the present study, 3V-cQFR would be rather practical compared to 3V-FFR, because FFR measurement is invasive and needs considerable time and cost for three-vessel analysis. In addition, cQFR could be calculated in vessels in which FFR could not be determined or might be associated with the potential risk related to wiring, such as with tortuous or calcified lesions. Advances in technology could overcome this issue in part, although not for all lesions. This could further highlight the potential clinical application of cQFR.
Some previous studies have failed to show the superiority of a revascularisation strategy over optimal medical therapy in stable lesions12,13. In the present study also, vessels with cQFR ≤0.80 did not have good prognosis regardless of revascularisation. Although this information is useful for clinical decision making, risk stratification for FFR-negative lesions would be needed because clinical events occur even in patients with non-ischaemic FFR values. Lee et al proposed that coronary physiological properties such as coronary flow reserve and index of microvascular resistance might be linked to an increased event rate in patients with physiologically non-significant stenosis14. The present study also presented the prognostic implication using 3V-cQFR in patients without functionally significant epicardial stenosis. This wireless, drugless method might be useful and practical for predicting future cardiovascular events in various subsets of patients. Further studies are warranted to investigate the utility of 3V-cQFR on MACE prediction and its influence on treatment decisions in a larger cohort.
Limitations
Because this study was an observational study at a single centre, it cannot escape selection bias. The assessment of 3V-cQFR was not achieved in approximately 20% of the enrolled patients, who could have developed adverse cardiovascular events. This is the inherent limitation of 3V-cQFR, while, simultaneously, QFR methods allowed us to investigate three-vessel physiological analyses retrospectively in 80% of the patients. 3V-cQFR was not compared to imaging-derived plaque burden or characteristics in this study, and could not represent the total plaque burden, especially for those who had diseased side branches, because side branches were not analysed. This study included some vessels without visual stenosis in order to enrol patients consecutively while the clinical utility of FFR or cQFR was investigated only for vessels with intermediate lesions. Moreover, the majority of cQFR computation required manual correction for the tracing (at least in part), which might have reduced the study’s generalisability. The limitations of using cQFR instead of FFR also include the exclusion of lesions with ostial stenosis or side branch lesions. Based on the acceptable agreement between FFR and cQFR, comparing the advantages of using FFR or cQFR is worth investigating in a large prospective clinical trial.
Non-adherence to medical therapy could be a selection bias, which could not be fully investigated. Clinical outcomes were mainly driven by ischaemia-driven revascularisation, although the revascularisation strategy was consistent in the present study. Further studies are needed to determine the prognostic impact of 3V-cQFR using hard endpoints in a prospective large-scale cohort. Nevertheless, the present 3V-cQFR could provide powerful prognostic information and might be a promising indicator of total atherosclerotic burden because the analyses need only angiograms.
Conclusions
A decrease in 3V-cQFR was independently associated with an increased incidence of MACE in patients with stable CAD, and the prognostic implication was more powerful than conventional angiographic scores. 3V-cQFR provided a prognostic implication in patients without functionally significant stenosis. 3V-cQFR could highlight total atherosclerotic burden, potentially relevant to future cardiovascular events.
|
Impact on daily practice Contrast-flow quantitative flow ratio (cQFR) is well correlated with fractional flow reserve (FFR), which can be calculated from angiograms. This study for the first time showed the prognostic implication of the sum of cQFR in three vessels (3V-cQFR), which was more powerful than that of conventional angiographic scores. Compared to FFR measurement, which is invasive, costly, time-consuming and can cause discomfort, 3V-cQFR would be practical in clinical settings. |
Acknowledgements
We thank all the physicians, nurses, other catheter laboratory staff members, and patients involved in this study.
Conflict of interest statement
The authors have no conflicts of interest to declare.

