While humans may base their decision-making on dichotomous threshold values, natural forces are typically on a continuum. Johnson et al1 demonstrated a continuous relationship between the fractional flow ratio (FFR) and a lesion’s ischaemic potential and prognosis. The lower the FFR, the more major adverse cardiovascular events (MACE) occurred over time. It follows that the greater the impairment of FFR, the greater the benefit of revascularisation (principally by percutaneous coronary intervention [PCI]) compared to medical therapy2.
We have entered an era of novel physiological measurements which estimate FFR from coronary angiography. The quantitative flow ratio (QFR; Medis Medical Imaging Systems) approximates the invasive pressure wire-based FFR. It has completed initial validation studies34 and is now being applied in large clinical trials, evaluating its performance against FFR as well as outcomes in common clinical scenarios. In this issue of EuroIntervention, Guan et al5 logically ask whether QFR, like FFR, will also be a prognosticator of post-PCI major adverse events.
Using patients from the FAVOR III China3 (5,564 vessels) and PANDA III4 trials (4,471 vessels), QFR was measured offline in vessels with a reference diameter ≥2.5 mm and at least 1 intermediately narrowed lesion (50-90% diameter stenosis). At 2-year follow-up, when compared with medical therapy, PCI reduced the risk of myocardial infarction (MI) in vessels with a QFR ≤0.80 (3.0% vs 4.6%) but increased the MI risk in vessels with a QFR>0.80 (3.6% vs 1.2%). Furthermore, QFR showed a significant, continuous, inverse association with spontaneous MI, which was reduced more with PCI than with medical therapy (p<0.0001). Lastly, the interaction statistics indicated a net benefit for PCI over medical therapy to reduce total MI (both spontaneous and periprocedural), beginning at QFR=0.64, a value very close to Johnson et al’s 0.67 FFR threshold1 (Figure 1).
Guan et al5 conclude that while an initial strategy of PCI led to more ischaemia-driven target vessel revascularisation and more periprocedural myocardial infarctions, PCI maintained clinical advantages over medical therapy: 1) vessels with a QFR ≤0.64 had fewer total MI after PCI, on a continuum of a decreasing QFR value and 2) PCI improved angina for patients with a low QFR, just as has been shown for numerous FFR trials6. In other words, vessels with lower QFR values had a proportionally larger reduction in their MI risk. It is important to note that the same ischaemic continuum was not observed for QFR and quantitative angiographic percentage diameter stenosis. This disparity between QFR values and anatomical narrowings is consistent with the visual-functional (e.g., FFR) mismatch commonly seen during coronary angiography and is the fundamental rationale for using physiology.
There are several issues to consider before accepting QFR as a replacement for FFR in daily practice. While large and well conducted, this study5 uses pooled trial data to make observations that were not prespecified. Treatments were not randomised on a per-vessel basis due to both the existing different protocols of FAVOR III China and PANDA III34 and lack of operator compliance. In PANDA III4, a prospective multicentre registry of 2-stent platforms, there appeared to be no medical therapy arm. In FAVOR III China3, optimal medical therapy alone was prescribed in patients without PCI. In a retrospective analysis of pooled data, one might find an uneven patient distribution affecting propensity score matching of patients and vessels for a specific treatment. Also lacking were detailed medication information, quantitative angina scores, and in many patients, invasive FFR testing. Some clinical events could not be attributed to a specific vessel, as repeat angiography was not routinely performed.
There are also technical limitations to QFR (as well as to any angio-FFR systems). Guan et al5 noted that QFR could not be computed in 20% of vessels. The principal reasons for this are likely the quality of the angiograms, the unavoidable artefacts of vessel overlap, contrast streaming, tortuosity, and panning, to name just a few. In addition, the algorithms for QFR use a three-dimensional model, incorporating various geometric parameters, such as vessel diameter, lesion length, and minimum lumen diameter, then applying computational fluid dynamics principles and blood flow characteristics, including estimated pressure drop and flow velocity which are subject to variations impacting the calculation. In addition, the accurate estimation of microvascular resistance appears to be among the most significant factors causing discordance between angiographic- and wire-based FFR7. In a comparison study of 5 different angio-FFR systems7, the false positive rates for a QFR <0.80 with an FFR>0.80 were often associated with the left anterior descending coronary artery, large vessel size, and increased microvascular resistance, while a false negative (QFR>0.80 with FFR <0.80) association more often involved the right or circumflex coronary vessels or small vessel sizes. Ninomiya et al7 suggested that the diagnostic accuracy previously reported in validation studies requires confirmation in large clinical trials, although not every available system was tested8.
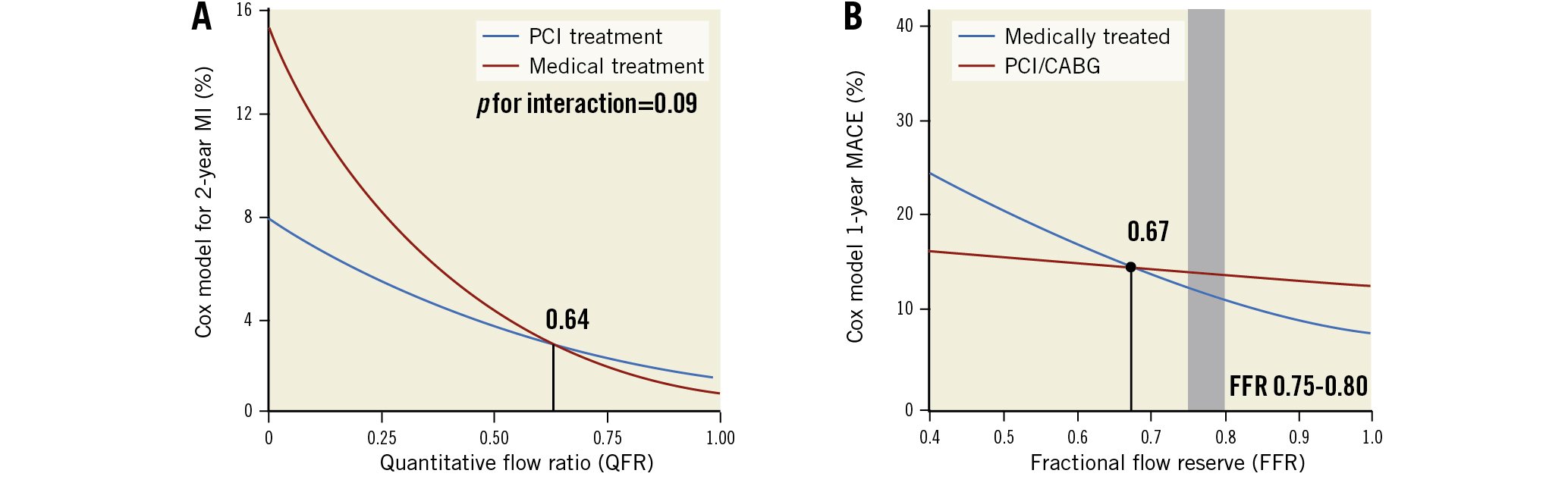
Figure 1. Revascularisation for coronary artery stenosis. Revascularisation for coronary artery stenosis for patients with a low FFR (<0.645, <0.671) was associated with better outcomes than for medical treatment, whereas for a stenosis with a high FFR (≥0.67), medical treatment would be a reasonable and safe treatment strategy. A) from Guan et al5, B) with permission, from Johnson et al1. CABG: coronary artery bypass graft; MACE: major adverse cardiovascular event; MI: myocardial infarction; PCI: percutaneous coronary intervention
The bottom line
Guan et al5 have increased our confidence in the angio-FFR applications, by informing us that QFR, like FFR, can predict risk for coronary artery disease patients. The question most often asked now is, “Can QFR replace invasive wire-based FFR?” Based on the available studies, as well as the logistical challenges of FFR measurement and the cost of the sensor wire, the answer is yes. QFR is less accurate but may cost less and, uniquely, is able to perform lesion assessment both prospectively and retrospectively from any well-done angiogram. This technique is simpler, safer, and less expensive with equivalent outcomes and will conceivably be readily and widely adopted. Angiographically derived FFR, and specifically QFR, should, in the future, be used routinely, reserving the invasive wire-based FFR approach for the challenging minority of cases where QFR accuracy is questioned.
Conflict of interest statement
M.J. Kern is aspeaker for Abbott Vascular, Philips, ACIST Medical Systems, Opsens, CathWorks, and ZOLL Medical.

