Abstract
Background: Quantitative flow ratio (QFR) is a novel angiography-based physiological index for fast computation of fractional flow reserve without the use of a pressure wire or induction of hyperaemia.
Aims: We sought to investigate the prevalence and prognostic implications of achieving physiology-consistent percutaneous coronary intervention (PCI) according to the baseline angiographic QFR in an all-comers cohort.
Methods: QFR was retrospectively analysed from the angiograms of 1,391 patients enrolled in the randomised PANDA III trial. Patients in whom all functionally ischaemic vessels (baseline QFR ≤0.80) were treated and in whom all non-ischaemic vessels (baseline QFR >0.80) were deferred were termed as having had QFR-consistent treatment; otherwise, they were termed as having had QFR-inconsistent treatment. The major outcome was two-year major adverse cardiac events (MACE; a composite of all-cause death, all myocardial infarction (MI), or any ischaemia-driven revascularisation).
Results: Overall, 814 (58.5%) patients had QFR-consistent PCI, while 577 (41.5%) patients received QFR-inconsistent PCI. Patients with QFR-consistent versus those with QFR-inconsistent treatment had a lower risk of two-year MACE (8.4% vs 14.7%; hazard ratio [HR] 0.56, 95% confidence interval [CI]: 0.41-0.78). After adjusting for differences in baseline covariates, two-year rates of MACE remained significantly lower in the QFR-consistent group (8.8% vs 13.6%; adjusted HR 0.64, 95% CI: 0.44-0.93), due mainly to reduced ischaemia-driven revascularisation (2.9% vs 8.0%; adjusted HR 0.35, 95% CI: 0.20-0.60).
Conclusions: In this post hoc analysis of an all-comers PCI trial, approximately 60% of patients were treated in accordance with what the QFR measurement would have recommended, the achievement of which was associated with improved two-year clinical outcomes. ClinicalTrials.gov identifier: NCT02017275
Introduction
Whether percutaneous coronary intervention (PCI) reduces death or myocardial infarction (MI) in patients with stable coronary artery disease (CAD) remains controversial. The ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trial showed that, compared with an initial conservative strategy of optimal medical therapy (OMT), an initial invasive strategy did not improve the prognosis of patients with stable CAD (other than relieving angina)1. In contrast, the long-term prognosis of patients undergoing fractional flow reserve (FFR)-guided PCI was improved compared with angiography-guided PCI in the earlier FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) trial2 and compared with OMT in the FAME 2 (Fractional Flow Reserve versus Angiography for Multivessel Evaluation 2) trial3, suggesting that physiology-guided assessment might identify patients and lesions that could potentially benefit from PCI. However, concerns about prolonged procedural time, side effects from pressure wire equipment, and hyperaemia induced by vasodilator medications have limited the widespread adoption of pressure wire-based physiological assessment (e.g., FFR, instantaneous wave-free ratio [iFR] and others)4.
Quantitative flow ratio (QFR) is a novel angiography-based physiological index that has been validated as having good reproducibility and diagnostic accuracy in identifying physiologically significant coronary stenoses compared with FFR as the reference standard5. However, there is a lack of knowledge regarding how frequently QFR-consistent revascularisation is achieved, and whether this criterion is associated with an improved prognosis. Therefore, in the present study we analysed the performance of PCI according to the baseline QFR of patients enrolled in the all-comers randomised PANDA III (Comparison of BuMA eG Based BioDegradable Polymer Stent With EXCEL Biodegradable Polymer Sirolimus-eluting Stent in “Real-World” Practice) trial (NCT02017275)6, and examined the outcomes of PCI according to QFR-based stratification.
Methods
PANDA III trial and the present study
The present study was a post hoc analysis from the PANDA III trial in which QFR analysis was retrospectively performed. PANDA III was a multicentre trial with few exclusion criteria in which 2,348 patients were randomised to two biodegradable polymer-based sirolimus-eluting stents with differing elution and absorption kinetics. In this all-comers cohort, patients underwent angiography-guided PCI; physiological assessment (including FFR and iFR) was infrequently used, and QFR was not available to the operators. The one-year and two-year rates of major adverse cardiac events (MACE) were similar with both stent types67. Data from the two arms were pooled for the present analysis. The PANDA III trial and the present study were approved by an institutional review committee and all subjects provided written informed consent.
Measurement of QFR
For the present analysis, QFR assessment was retrospectively performed in all eligible vessels, defined as those containing lesions with ≥50% diameter stenosis (DS) and with reference vessel diameter (RVD) ≥2.5 mm by visual assessment. Off-line QFR analysis was performed by technicians at an independent core laboratory (CCRF, Beijing, China), blinded to clinical outcomes using a QFR system (AngioPlus; Pulse Medical Imaging Technology, Shanghai, China). QFR analysis was performed following a standard operation procedure, as previously reported58, the details of which are described in Supplementary Appendix 1. QFR has been well validated against FFR as the reference standard5; the cut-off value of QFR for physiological significance has been established as 0.80, which is also being used in the ongoing FAVOR III China trial (NCT03656848)8 and FAVOR III EJ trial (NCT03729739).
Quantitative coronary angiography (QCA) and SYNTAX score
QCA characteristics, including the RVD, minimal lumen diameter (MLD), DS% and lesion length, were analysed at the core laboratory using well-validated software (QAngio version 7.3; Medis Medical Imaging Systems, Leiden, the Netherlands). From the baseline or post-procedure angiograms, the SYNTAX score (SS) and residual SYNTAX score (rSS) were determined using an online calculator based on a specific scoring algorithm. The functional SYNTAX score (FSS) was calculated by summing only the individual SS of lesions with vessel QFR ≤0.809.
Stratification strategy
Vessels were defined as having physiologically significant ischaemia if the baseline QFR of the vessel was ≤0.80. As shown in Supplementary Figure 1, patients in whom all physiologically significant ischaemic vessels were treated by PCI and in whom all vessels with QFR >0.80 were deferred were termed as having had QFR-consistent treatment; otherwise, they were termed as having had QFR-inconsistent treatment. The QFR-inconsistent group was further stratified into three subgroups: 1) QFR-based undertreatment (QFR-UT; patients with at least one physiologically significant ischaemic vessel [baseline QFR ≤0.80] in which PCI was not performed and in whom all vessels that were physiologically non-ischaemic [baseline QFR >0.80] were also not treated); 2) QFR-based overtreatment (QFR-OT; patients with at least one physiologically non-ischaemic vessel [baseline QFR >0.80] in which PCI was performed and in whom all vessels that were physiologically significant ischaemic [baseline QFR ≤0.80] were also treated); and 3) QFR-based overtreatment and undertreatment (QFR-OUT; patients with at least one physiologically significant ischaemic vessel not treated and at least one physiologically non-ischaemic vessel treated).
Endpoints and follow-up
The primary outcome for the present study was the two-year rate of MACE (defined as the composite of all-cause death, all MI, or any ischaemia-driven revascularisation). Secondary outcomes included the individual components of MACE and stent thrombosis. All definitions of clinical endpoints were identical to the PANDA III trial6. Detailed endpoint definitions are provided in Supplementary Appendix 2. All adverse events were adjudicated by a clinical events committee blinded to QCA and QFR analyses.
Statistical analysis
Baseline characteristics and two-year clinical outcomes were compared in patients stratified according to their post-procedural QFR-consistent versus QFR-inconsistent status. Continuous variables are expressed as mean±SD or median (interquartile range [IQR]) and were compared using the Student’s t-test or the Mann-Whitney U test, as appropriate. Categorical variables are presented as counts (%) and were compared using the chi-square test or Fisher’s exact test, as appropriate. The cumulative incidence of clinical events is presented as Kaplan-Meier estimates. The Cox proportional hazards model was used to estimate the HR and 95% CI. Confounding due to differences in baseline characteristics was addressed using two propensity analysis methods (inverse probability of treatment weighting [IPTW] and propensity score matching [PSM]). Standardised mean differences (SMD) were used to assess the balance between the groups, with a standardised difference of 10% or less deemed to be an excellent balance and a standardised difference of 20% or less deemed to be an acceptable balance. The details of IPTW and PSM are described in Supplementary Appendix 3. Unless otherwise specified, a two-sided p-value <0.05 was considered to indicate statistical significance. Database management and data analyses were performed by an independent clinical research organisation (CCRF, Beijing, China). All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients and QFR stratification
Among the 2,348 patients enrolled in the PANDA Ⅲ trial, 957 patients in whom QFR in at least one stenotic vessel was unanalysable were excluded, mostly due to the absence of calibration data in the DICOM files (Figure 1). Details of the exclusion criteria are shown in Supplementary Appendix 1. Therefore, 1,391 patients (59.2%) were included in the present study. The baseline characteristics of the included and excluded cohorts are shown in Supplementary Table 1. The excluded patients were more likely to possess features reflecting lower measurability of patient-level QFR analysis (e.g., multivessel CAD, left main lesion) and a higher incidence of comorbidities. The two-year MACE rates were similar between these two groups (Supplementary Table 2).
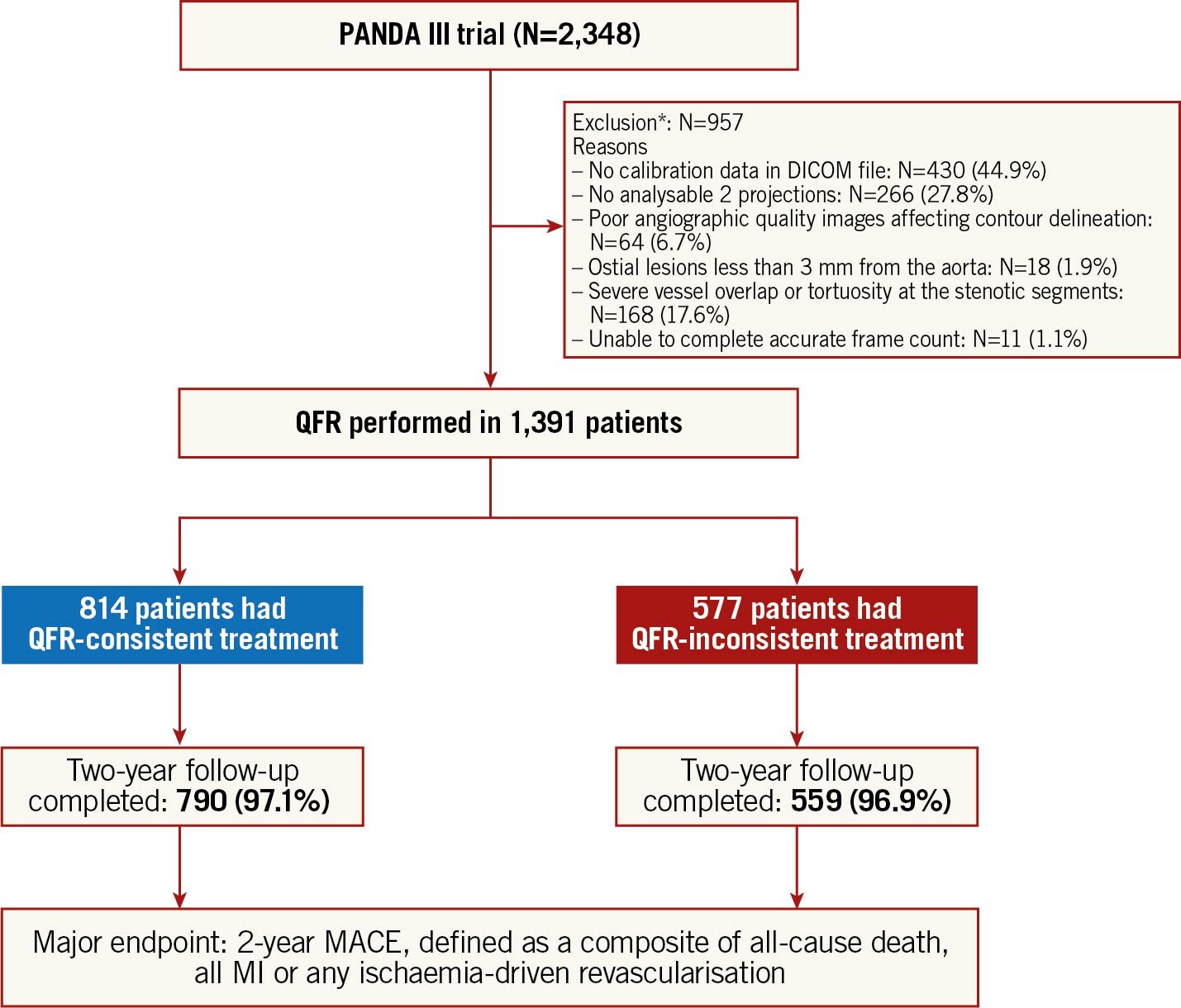
Figure 1. Study flow chart. *A hierarchical listing based on the processes for QFR assessment was used to identify the exclusion reasons per patient. DICOM: Digital Imaging and Communication in Medicine; MACE: major adverse cardiac events; MI: myocardial infarction; N: number of patients; QFR: quantitative flow ratio
Among the 1,391 study patients, QFR was assessed in 2,543 vessels (3,017 lesions) and PCI was performed in 1,717 vessels (1,980 lesions). QFR-consistent treatment was performed in 814 (58.5%) patients while 577 (41.5%) had QFR-inconsistent treatment, including 344 (24.7%) QFR-UT, 205 (14.7%) QFR-OT, and 28 (2.0%) QFR-OUT. Details of the QFR-based physiological assessment are presented in Figure 2.
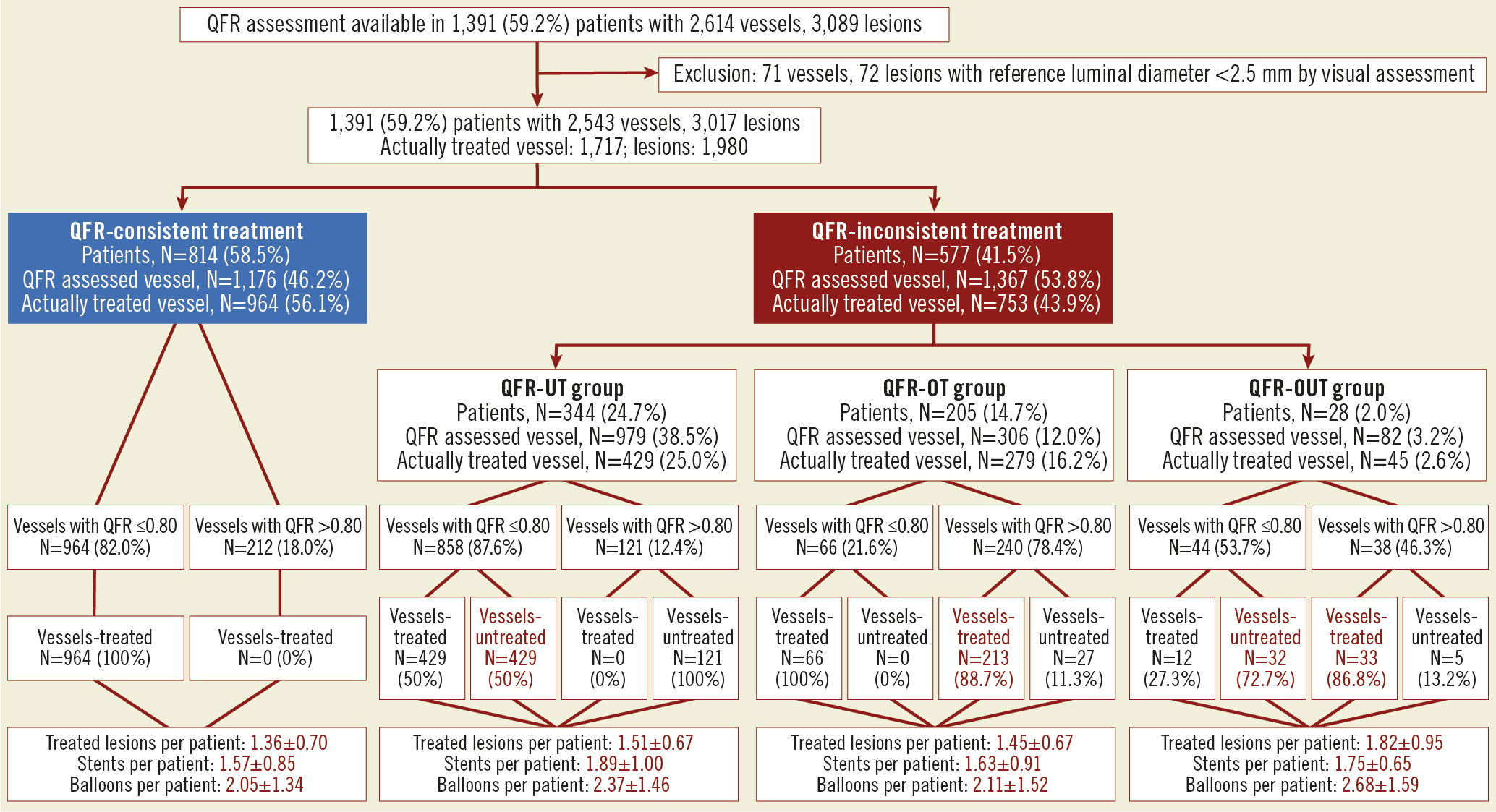
Figure 2. Incidence of QFR-based physiological revascularisation. N: number of patients; QFR: quantitative flow ratio; QFR-OT: QFR-based overtreatment; QFR-OUT: QFR-based overtreatment and undertreatment; QFR-UT: QFR-based undertreatment
Baseline characteristics
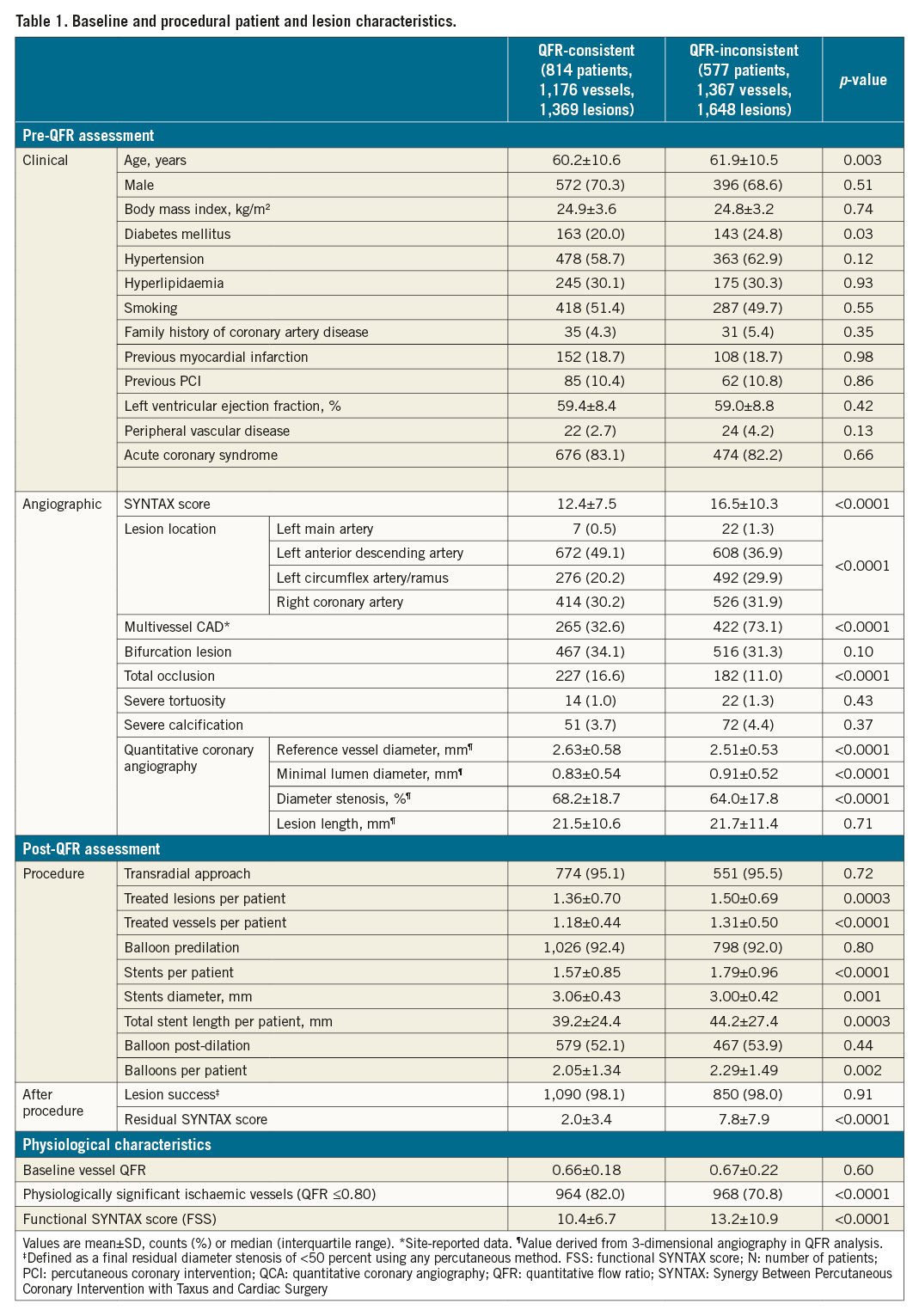
Baseline characteristics of the QFR-consistent and QFR-inconsistent groups are summarised in Table 1. Patients with QFR-inconsistent treatment were more likely to have multivessel CAD, a higher SYNTAX score and coexisting comorbidities (e.g., older age, diabetes mellitus). Patients with QFR-inconsistent treatment also had more lesions treated with greater numbers of stents and a higher residual SYNTAX score. Moreover, baseline vessel QFR in the QFR-consistent group was comparable to the QFR-inconsistent group, with a higher proportion of physiologically significant ischaemic vessels and lower FSS. The baseline characteristics of the four subgroups (QFR-consistent, QFR-UT, QFR-OT, and QFR-OUT) are presented in Supplementary Table 3.
Two-year clinical outcomes (unadjusted)
The Kaplan-Meier estimates for two-year MACE were 8.4% and 14.7% in the QFR-consistent and QFR-inconsistent groups, respectively; HR 0.56, 95% CI: 0.41-0.78 (Supplementary Figure 2A). The differences in MACE between groups were driven principally by fewer ischaemia-driven revascularisations in the target and non-target vessels (3.0% vs 8.6%; HR 0.35, 95% CI: 0.22-0.56; p<0.0001) (Supplementary Figure 2D). The rates of other individual components of MACE and stent thrombosis are presented in Table 2 and Supplementary Figure 2. The two-year relative rates of MACE were consistent across the examined major subgroups (Supplementary Figure 3). In addition, Figure 3 shows the Kaplan-Meier estimates for two-year MACE and its individual components in the QFR-consistent, QFR-OT, QFR-UT, and QFR-OUT groups. The cumulative incidences of two-year MACE were 8.4%, 8.3%, 18.5% and 14.3% in the QFR-consistent, QFR-OT, QFR-UT, and QFR-OUT groups, respectively.
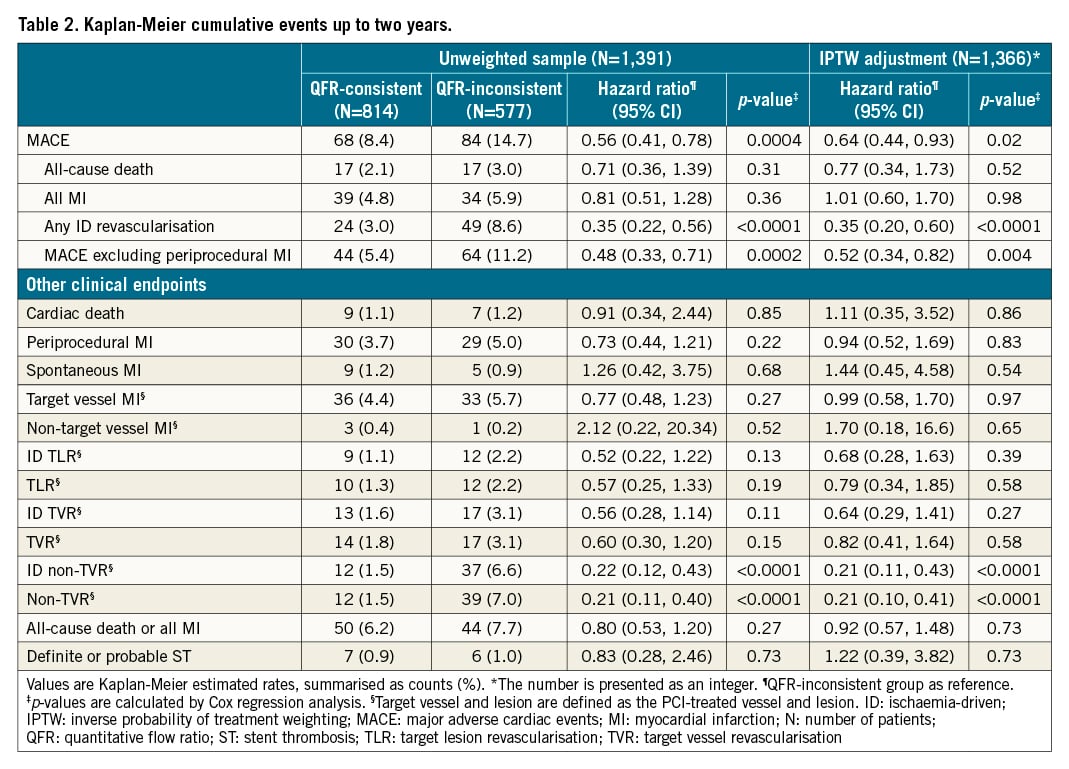
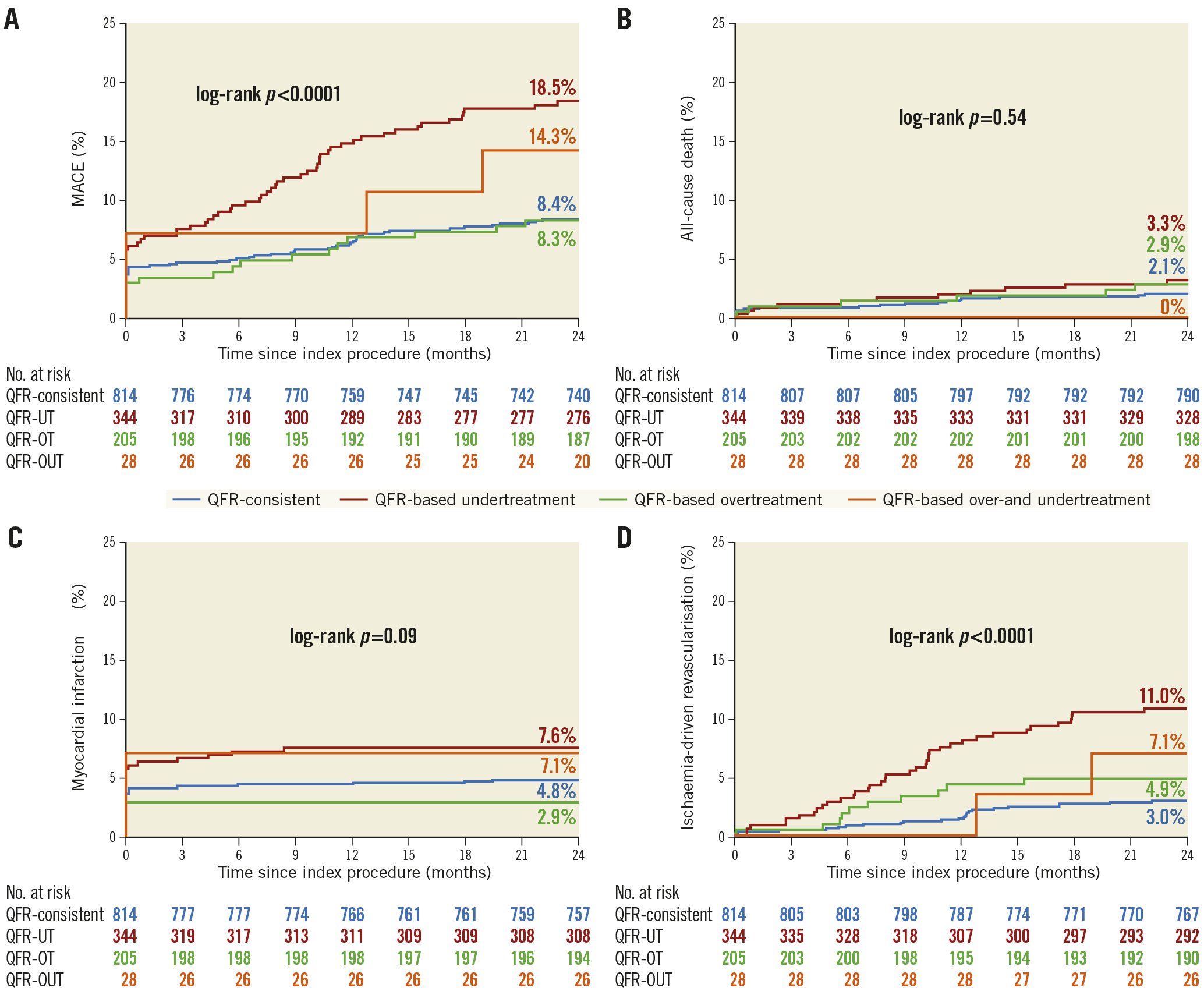
Figure 3. Time-to-event curves of two-year clinical outcomes among the QFR-consistent, QFR-UT, QFR-OT, and QFR-OUT groups. Kaplan-Meier time-to-first-event curves showing the two-year cumulative incidence of the following. A) Major adverse cardiac events (MACE). B) All-cause death. C) All myocardial infarction. D) Ischaemia-driven revascularisation. CI: confidence interval; HR: hazard ratio; MACE: major adverse cardiac events; QFR-OT: QFR-based overtreatment; QFR-OUT: QFR-based overtreatment and undertreatment; QFR: quantitative flow ratio; QFR-UT: QFR-based undertreatment
Inverse probability of treatment weighting analysis
The area under the curve (AUC) of the propensity model and the distribution of propensity scores are presented in Supplementary Figure 4 and Supplementary Figure 5. IPTW, based on propensity score, resulted in excellent or acceptable between-group balance on most pre-QFR-assessment baseline characteristics (Supplementary Table 4), although left anterior descending (LAD) lesions (SMD, 0.113), multivessel CAD (SMD, 0.113) and DS (SMD, 0.114) were slightly higher among QFR-inconsistent patients.
After IPTW, the two-year rates of MACE (8.8% vs 13.6%; adjusted HR 0.64, 95% CI: 0.44-0.93; p=0.02) (Figure 4A) and ischaemia-driven revascularisation (2.9% vs 8.0%; adjusted HR 0.35, 95% CI: 0.20-0.60; p<0.0001) remained significantly lower in the QFR-consistent group than in the QFR-inconsistent group (Figure 4D). Comparison of other adverse events is shown in Figure 4 and Table 2.
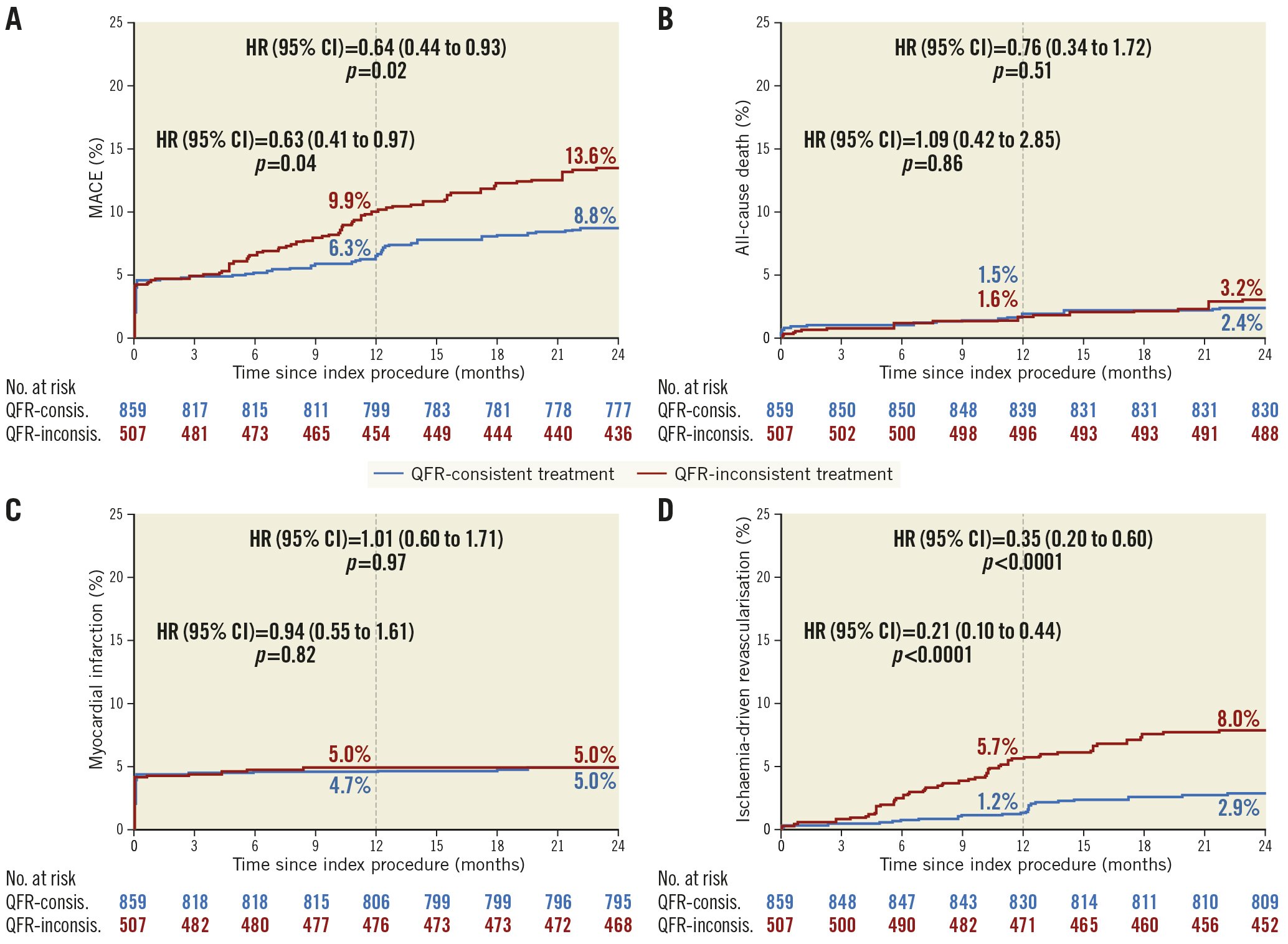
Figure 4. Time-to-event curves of two-year clinical outcomes by QFR-consistent and QFR-inconsistent groups after IPTW. Kaplan-Meier time-to-first-event curves showing the two-year cumulative incidence of the following. A) MACE. B) All-cause death. C) Any myocardial infarction. D) Ischaemia-driven revascularisation. The numbers are presented as integers. CI: confidence interval; HR: hazard ratio; IPTW: inverse probability of treatment weighting; MACE: major adverse cardiac events; QFR: quantitative flow ratio
Sensitivity analyses
Sensitivity analysis was performed comparing the characteristics and outcomes between the QFR-consistent group and the QFR-UT group after 1:1 propensity matching. The AUC of the propensity model, the distribution of propensity scores, and between-group baseline characteristics are presented in Supplementary Table 5, Supplementary Figure 6 and Supplementary Figure 7. The QFR-consistent group was associated with lower two-year MACE compared with the QFR-UT group in both unadjusted and matched samples (unadjusted sample, HR 0.43, 95% CI: 0.31-0.61; matched sample, adjusted HR 0.55, 95% CI: 0.33-0.92) (Supplementary Table 6, Supplementary Figure 8).
In a second sensitivity analysis, a comparison between the QFR-consistent group and the QFR-OT group was performed. Supplementary Table 7, Supplementary Figure 9 and Supplementary Figure 10 show the AUC of the propensity model, the distribution of propensity scores, and the between-group baseline characteristics. The difference between the QFR-consistent group and the QFR-OT group in two-year adverse events was not statistically significant (Supplementary Table 8, Supplementary Figure 11). However, in a PSM analysis, fewer stents and balloons were used in the QFR-consistent group than in the QFR-OT group (stents per patient, 1.52 vs 1.75, p=0.02; balloons per patient, 2.02 vs 2.37, p=0.02).
The rationale for the decision-making of treating vessels on the basis of angiography was investigated in a third sensitivity analysis (Supplementary Table 9). In vessels with QFR ≤0.80, non-LAD lesions with smaller reference vessel diameter and with lesser anatomical complexity (e.g., low vessel SYNTAX score, non-occlusion, low DS%) were more likely to be deferred. In contrast, among vessels with QFR >0.80, LAD lesions with larger reference vessel diameter and with greater anatomical complexity (e.g., high vessel SYNTAX score) were more likely to be treated.
Discussion
The main findings of the present retrospective analysis in which patient-level baseline QFR assessment was feasible in 1,391 patients from the all-comers randomised PANDA III trial are: 1) according to retrospective QFR analysis of the all-comers PANDA III trial, angiography-guided PCI did not address flow-limiting disease correctly in a large number of patients (41%); 2) QFR-consistent treatment was associated with a lower risk of two-year MACE compared with the QFR-inconsistent group, with differences driven by fewer ischaemia-driven revascularisations during follow-up; and 3) after accounting for differences in baseline covariates between the groups, being QFR-consistent was an independent predictor of freedom from two-year MACE (Central illustration).
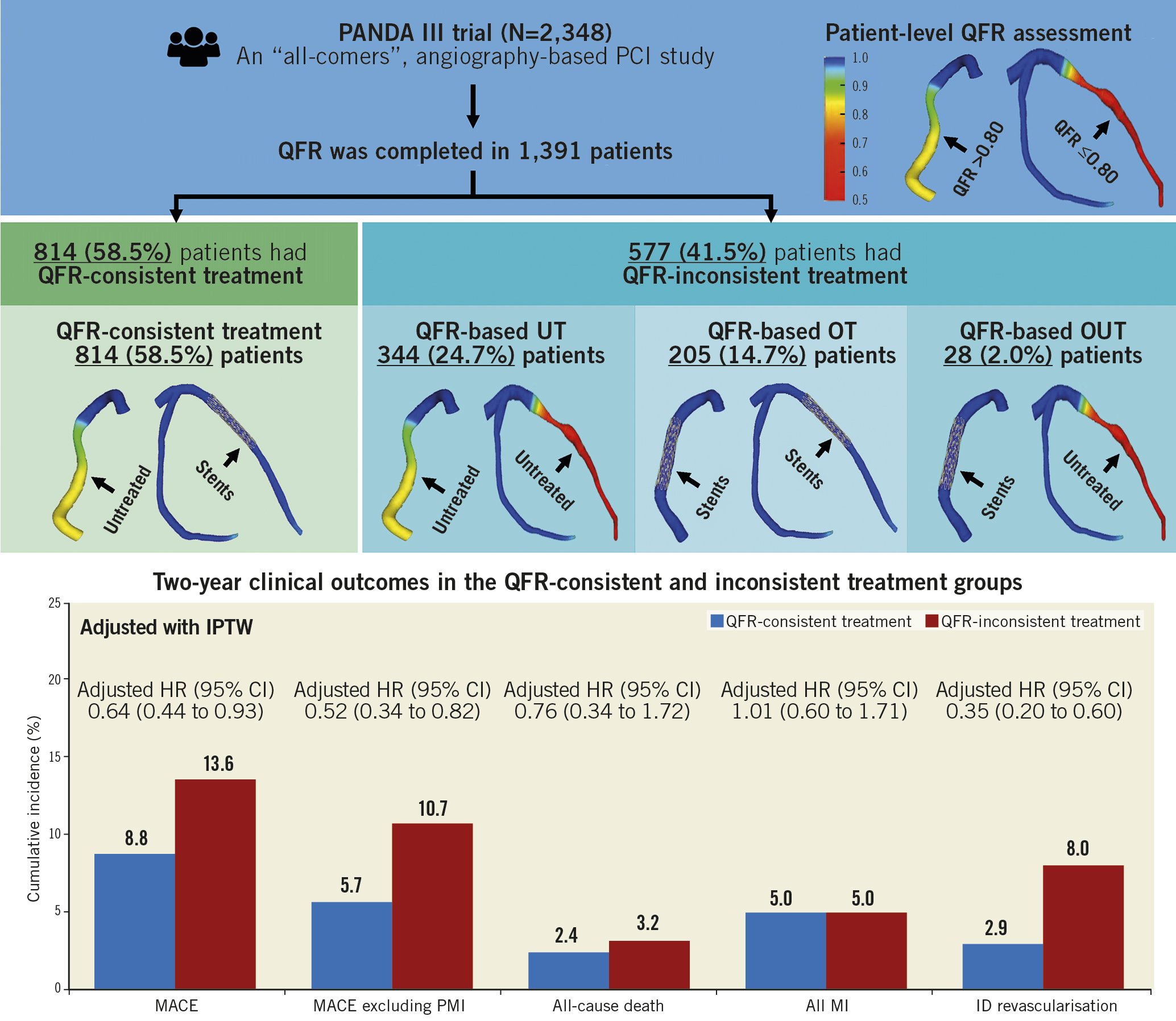
Central illustration. QFR-based stratification and two-year outcomes. CI: confidence interval; HR: hazard ratio; ID: ischaemia-driven; IPTW: inverse probability of treatment weighting; MACE: major adverse cardiac events; OT: overtreatment; OUT: overtreatment and undertreatment; PMI: periprocedural myocardial infarction; QFR: quantitative flow ratio; UT: undertreatment
Patient-level QFR assessment was available in 59.2% of patients. The main reason for unavailable QFR was the lack of calibration data in DICOM files (44.9%). There are several prerequisites for a DICOM file to be analysed by the current QFR system, namely three DICOM tags, i.e., 1) tag ID (0018,1110), 2) tag ID (0018,1111), and 3) tag ID (0018,1164); DICOM files were not analysable if any of the three parameters were missing, which may be related to the angiography mode or the angiography device model. In addition, in the PANDA III trial, without specific angiographic guidelines, the lack of analysable projections (27.8%) and severe vessel overlap or tortuosity (17.6%) were also important reasons for patient exclusion. The availability of QFR can be improved by specifying appropriate equipment and modes before angiography and following specific angiographic guidelines.
QFR has been developed as a simple-to-use adjunct to angiography and PCI which enables physiological lesion and vessel assessment without the need for pressure wire measurement of non-standard hyperaemic agents5. Its simplicity, shorter assessment times, and fewer complications compared to FFR may promote the routine online use of this technique to assist the decision making of interventionalists4. However, while QFR has been shown to have very good correlation with FFR5, no randomised trials have compared clinical outcomes of QFR-based and FFR-based or iFR-based revascularisation decisions. Also, no prior studies have assessed what proportion of patients undergoing angiography-guided PCI achieve functionally consistent revascularisation according to QFR indices, and whether such categorisation might be of prognostic utility. In this post hoc analysis from an all-comers randomised trial in which PCI was performed with contemporary drug-eluting stents (DES), we found that ~59% of patients achieved QFR-consistent physiological revascularisation – that is, all vessels with a baseline QFR at or below the ischaemic threshold of 0.80 were treated and no vessels with a QFR above this threshold were treated. Conversely, ~41% of patients received QFR-inconsistent physiological revascularisation, of whom approximately 60% were undertreated and 36% were overtreated. After adjusting for differences in clinical and angiographic covariates, MACE rates up to two-year follow-up were lower if QFR-based treatment guidance had been followed. These findings suggest that an angiographic QFR-guided revascularisation strategy may improve clinical outcomes of patients undergoing PCI.
QFR-consistent treatment was associated with lower one-year and two-year rates of MACE, with the hazard curves continuing to diverge with longer-term follow-up, as reported in previous studies31011. The improved late prognosis of QFR-consistent treatment was due mostly to the reduction of ischaemia-driven revascularisation, with differences noted especially in the non-target vessel but also in the target vessel. Although the differences in all-cause death or all MI were not significant between the groups, numerically fewer events occurred in the QFR-consistent group. Examining the differences between treated and untreated vessels, it was observed that the interventionalists (using angiographic guidance) tended to treat LAD lesions of greater anatomic complexity more frequently than right coronary artery (RCA) or left circumflex (LCx)lesions of lesser complexity. However, this is a suboptimal manner to identify physiologically significant ischaemia. Therefore, vessels truly in need of angioplasty could be effectively identified by physiological assessment, which has been demonstrated to improve the prognosis of patients undergoing PCI in the present study.
Sensitivity analysis demonstrated that the worse outcomes in the QFR-inconsistent group compared with the QFR-consistent group were driven most strongly by adverse outcomes in the QFR-UT group, indicating that contemporary DES treatment of vessels with QFR-based physiologically significant ischaemia is especially important to improve clinical outcomes (consistent with the findings from FAME 2 and other studies using FFR-based physiological assessment)381213. Patients with a deferred functional ischaemia vessel (baseline QFR ≤0.80) were more likely to suffer from recurrent angina, which means a higher possibility of undergoing coronary angiography again and further intervention on the deferred vessels. This might explain the fact that the improved late prognosis of QFR-consistent treatment was mostly due to the reduction of ischaemia-driven revascularisation, especially in the non-target vessel. Compared with the QFR-OT group, no significant long-term clinical benefits were observed in the QFR-consistent group, consistent with the DEFER study outcomes1415 and the long-term clinical results from the FAME trial2. However, by avoiding treatment of vessels that are physiologically non-ischaemic, QFR-consistent treatment compared with QFR-OT may reduce the per-patient use of interventional devices (e.g., stents, balloons), thereby lowering the cost of medical care and the risk of procedural complications, as was observed in the economic evaluation of the FAME study16. Nevertheless, overtreatment of physiologically insignificant vessels might theoretically be related to adverse events such as periprocedural MI, stent thrombosis, in-stent restenosis, and target lesion revascularisation, detection of the differences which depend on a large sample size. In the present retrospective analysis, as the sample size was limited (QFR-OT, N=205), there was not enough power to test differences in these events, so the findings of this study are hypothesis generating and need a future massive prospective randomised trial for validation.
In the present study, by retrospective off-line QFR analysis of an all-comer cohort undergoing angiography-guided PCI, we sketched a picture that almost 59% achieved physiology-consistent PCI, while 41% received physiology-inconsistent PCI. These findings revealed the net benefits and rationale behind the improved clinical prognosis of physiology-guided revascularisation, as nearly 40% of the patients whose intervention strategies were guided by angiography could be converted to achieve functional revascularisation based on preprocedural physiology assessment. This supports the use of physiology-guided decisions in the catheter laboratory and lends confidence to clinicians to adopt this approach to optimise decision making. However, the findings of the present study are hypothesis generating, and the utility and cost-effectiveness of QFR-guided PCI compared with angiography guidance alone is being examined in 3,830 randomised patients in the ongoing prospective FAVOR III China trial (NCT03656848)8.
Limitations
A strength of the present study is that the large-scale all-comers PANDA III trial incorporated numerous high-quality measures, including on-site monitoring, clinical event adjudication and independent QCA analysis. However, PANDA III was not designed to facilitate QFR analysis. Thus, many of the angiographic images retrospectively analysed in the present study failed to meet the analysis requirements of the QFR analysing system, resulting in the exclusion of 40.8% of patients from the final analysis, which is in line with previous studies of retrospective QFR analysis9. Some baseline characteristics were not evenly distributed between included and excluded patients. The extent to which these considerations may affect the external validity of the present results is unknown. Also, patients in whom QFR-consistent treatment was achieved had fewer comorbidities and less extensive CAD than those with QFR-inconsistent treatment. Although the differences in outcomes favouring the QFR-consistent group persisted after adjusting for most of these imbalances, we cannot exclude an effect from unmeasured confounders. Third, a few patients received a planned staged procedure but exceeded the pre-defined staged window of the PANDA III trial6, the grouping of whom might have introduced selection bias. Fourth, as a post hoc analysis to investigate the impact of physiology consistency on PCI outcomes by using the data from a head-to-head DES trial, there might be some inherent bias: 1) the primary outcome of the present study was a composite of all-cause death, all MI, or any ID revascularisation, which is inconsistent with the original primary endpoint (composite of cardiac death, target vessel MI, or ischaemia-driven target lesion revascularisation [ID-TLR]) in the PANDA III trial; 2) during the follow-up of the PANDA III trial, as the treatment decision was unblinded with participating patients, those with deferred vessels/lesions were more likely to undergo repeat coronary angiography and further revascularisation procedures due to self-reported symptoms. Fifth, the post-PCI QFR was reported to have a substantial impact on long-term clinical outcome17. The prognostic value of the strategy combining guidance of pre-PCI QFR and assessment of post-PCI QFR will be investigated in future studies. Sixth, intracoronary imaging (e.g., optical coherence tomography [OCT], intravascular ultrasound [IVUS]) was used relatively infrequently in PANDA III. Although physiology is recommended principally for lesion selection whereas intracoronary imaging is relied upon more for stent optimisation, the extent to which the present results may have differed had a greater reliance on intracoronary imaging been used is uncertain. Finally, the present study did not include a control arm of angiography-guided or FFR-guided revascularisation.
Conclusions
In this post hoc analysis of an all-comers PCI trial, approximately 60% of patients were treated in accordance with the QFR measurement which would have been recommended, the achievement of which was associated with improved clinical outcomes during two-year follow-up. The utility of QFR to guide revascularisation of patients undergoing PCI is being tested in the ongoing prospective randomised FAVOR III China trial.
Impact on daily practice
The present study in which QFR was retrospectively assessed in 1,391 patients from the all-comers PANDA III randomised trial demonstrated that ~60% of patients undergoing angiography-based PCI had QFR-consistent functional revascularisation, the achievement of which was associated with improved two-year clinical outcomes. This study supports the use of physiology-guided decisions in the catheterisation laboratory and lends confidence to clinicians to adopt this approach to optimise decision making.
Funding
The PANDA III trial was sponsored by Sino Medical, Tianjin, China. As a post hoc analysis, the present study was supported by Beijing Municipal Science and Technology Project (grant number: Z191100006619107), Capital Health Development Research Project (grant number: 2020-1-4032), and Chinese Academy of Medical Sciences Clinical and Translational Medicine Research Fund (grant number: 2019XK320056).
Conflict of interest statement
G.W. Stone has received speaker or other honoraria from Cook, Terumo, Qool Therapeutics and Orchestra Biomed; has served as a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, Matrizyme, and Cardiomech; and has equity/options from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, and Valfix. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

