Abstract
Background: The quantitative flow ratio (QFR) identifies functionally ischaemic lesions that may benefit more from percutaneous coronary intervention (PCI) than from medical therapy.
Aims: This study investigated the association between QFR and myocardial infarction (MI) as affected by PCI versus medical therapy.
Methods: All vessels requiring measurement (reference diameter ≥2.5 mm and existence of at least one stenotic lesion with diameter stenosis of 50-90%) in the FAVOR III China (5,564 vessels) and PANDA-III trials (4,471 vessels) were screened and analysed for offline QFR. The present study reported clinical outcomes on a per-vessel level. Interaction between vessel treatment and QFR as a continuous variable was evaluated for the threshold of 2-year MI estimated by Cox proportional hazards model.
Results: Compared with medical therapy at 2 years, PCI reduced the MI risk in vessels with a QFR ≤0.80 (3.0% vs 4.6%) but increased the MI risk in vessels with a QFR>0.80 (3.6% vs 1.2%). Additionally, continuous QFR showed an inverse association with spontaneous MI (hazard ratio [HR] 0.89, 95% confidence interval [CI]: 0.79-0.99; p=0.04) that was reduced by PCI compared to medical therapy (HR 0.26, 95% CI: 0.17-0.40; p<0.0001). The interaction indicated a net benefit for PCI over medical therapy to reduce total MI beginning at QFR ≤0.64.
Conclusions: The present study demonstrated a continuous, inverse relationship between the QFR value of a vessel and its subsequent risk for MI, and PCI, compared to medical therapy, reduced this risk beginning at a QFR value of 0.64. These novel findings provide physicians with an angiographic tool for optimising vessel selection for PCI.
Introduction
A series of 5 randomised controlled trials (RCT) enrolling over 11,000 total subjects has demonstrated that percutaneous coronary intervention (PCI) reduces spontaneous myocardial infarction (MI) compared to modern medical therapy alone for stable lesions123. While this benefit does not translate into less cardiovascular death in patients without multivessel disease4, which is perhaps due to competing risks and our generally successful treatments for MI, it nevertheless raises the question of lesion selection. Namely, trials showing that PCI reduces the risk of MI have used a variety of inclusion criteria from angiographic severity2 to non-invasive stress testing3 and intracoronary pressure wire measurement of fractional flow reserve (FFR)1.
Angiography-based simulations of coronary physiology have recaptured clinical interest after a long period of dormancy and invasive assessment. Among several techniques, the quantitative flow ratio (QFR) stands out for its evidence base5. QFR offers negligible bias and reasonable imprecision versus FFR678; additionally, QFR has provided superior clinical outcomes versus angiographic assessment91011.
Bringing these two themes together, we sought to study the association between QFR and MI as affected by PCI versus medical therapy. Given that FFR displays a continuous relationship between its numerical value and prognosis12, we conjectured that QFR would provide a similar risk continuum for MI. To test our hypothesis, we pooled data from 2 large RCT that measured QFR and monitored clinical outcomes.
Methods
In brief, we combined individual-level patient data from the previously published FAVOR III China910 and PANDA-III1314 trials. Because QFR provides a vessel-specific diagnosis, and PCI treats an individual vessel, our analysis was performed at the vessel level. QFR was analysed offline and in a blinded fashion for every major coronary artery with a diameter stenosis ≥50%. The study was registered with PROSPERO (CRD42023401604).
Summary of pooled trials
Supplementary Table 1 contains detailed inclusion and exclusion criteria for these 2 trials. FAVOR III China was an investigator-initiated, multicentre, blinded, randomised, sham-controlled trial comparing clinical outcomes after PCI guided by QFR or standard angiography. A total of 3,825 adults (≥18 years) were enrolled from 26 hospitals in the People’s Republic of China. Patients presented with stable or unstable angina pectoris, or an MI at least 72 hours before screening. All subjects had an initial plan for PCI based on angiographic assessment, with at least 1 major coronary artery (≥2.5 mm reference vessel diameter) containing a visual diameter stenosis of 50-90%. Subjects were randomly assigned to a PCI strategy using either standard angiographic guidance or QFR. In the QFR-guided group, a local (“online”) measurement of QFR determined treatment: QFR ≤0.80 received PCI, whereas QFR>0.80 received medical treatment.
PANDA-III was a prospective, multicentre RCT comparing 2 stent platforms (both biodegradable polymers eluting sirolimus but with differing elution and absorption kinetics) in an all-comers population with few limitations. A total of 2,348 adults (≥18 years) were enrolled from 46 hospitals in the People’s Republic of China. Patients presented with chronic, stable ischaemic heart disease or acute coronary syndromes, including MI with or without ST-segment elevation. All subjects had at least 1 major coronary artery (2.5-4.0 mm reference vessel diameter) with a visual diameter stenosis >50%. PCI treatment was based on standard angiographic guidance.
In both trials, subjects received loading doses of aspirin 300 mg (starting at least 24 hours before PCI) and either clopidogrel 300-600 mg or ticagrelor 180 mg (at least 6 hours before PCI). After PCI, subjects received ≥100 mg of aspirin daily for an indefinite period and clopidogrel (75 mg once daily) or ticagrelor (90 mg twice daily) for at least 12 months. For subjects without PCI in the QFR-guided arm of FAVOR III China, optimal medical therapy alone was prescribed. Both trials were approved by the study sites and their local ethics committees or institutional review boards. All patients provided informed consent before participation.
Offline QFR measurement
QFR was measured as previously described9. Briefly, it accounted for the entire contoured segment from the ostium to the distal vessel. For bifurcation lesions, QFR assessed both the main vessel and its daughter branch. An analyst selected the angiographic sequence, then chose the starting frame (as contrast entered the vessel) and the final frame (as contrast reached the distal vessel). Dedicated QFR software (AngioPlus system; Pulse Medical) delineated the lumen contour and reference vessel diameter automatically, which could be adjusted manually by fixed proximal segment mode or normal mode. Contrast flow velocity was calculated using the Thrombolysis in Myocardial Infarction (TIMI) frame count. With these acquired parameters, the QFR value was then calculated.
In FAVOR III China, central (“offline”) QFR assessment was prospectively mandated for all enrolled vessels by a blinded and independent core laboratory (Core Medical, Beijing, People’s Republic of China). Offline QFR assessment was retrospectively performed in all vessels enrolled in PANDA-III by a blinded and independent core laboratory (CCRF, Beijing, People’s Republic of China)15. Vessels with a valid and successful QFR analysis in FAVOR III China and PANDA-III were pooled into a common database, along with associated clinical and procedural characteristics.
Endpoints
The primary endpoint was spontaneous (not periprocedural) MI during the next 24 months. Secondary endpoints, during 2 years of follow-up, included cardiac death, periprocedural and spontaneous MI, and ischaemia-driven target vessel revascularisation (ID-TVR), both immediate (as part of the initial procedure) and delayed (either a first PCI of a medically treated vessel or repeat PCI of an initially revascularised vessel). The detailed definitions of the endpoints are listed in Supplementary Table 2. Both the FAVOR III China and PANDA-III trials used the same definitions for cardiac death, ID revascularisation events and spontaneous MI. While PANDA-III used the Academic Research Consortium (ARC) definition for periprocedural MI, the FAVOR III China trial used the protocol definition (creatine kinase [CK]-MB >3x upper limit of normal [ULN] or troponin >21x ULN with absence of CK-MB). To eliminate the difference, we readjudicated periprocedural MI events in PANDA-III using the FAVOR III China trial's protocol definition.
In both the FAVOR III China and PANDA-III trials, clinical events were adjudicated by the independent clinical events committees blinded to the randomisation assignment. In order to re-adjudicate clinical events into vessel level, two interventional cardiologists (R. Zhang and Z. Qiao) blinded to baseline clinical and procedural details – including QFR – reviewed all adverse event-related documents including medical records, electrocardiographs, or angiograms. Those events lacking clear evidence of a culprit vessel were excluded. A total of 132 patients with 193 vessels were excluded from the present analysis, which included 36 cardiac deaths (36 patients with 63 vessels) and 28 MIs (28 patients with 38 vessels) that did not receive a follow-up angiography at the time of event, had ambiguous angiographic evidence to identify a specific culprit vessel or were related to vessels unavailable for QFR calculation at baseline. A total of 78 revascularisations (78 patients with 92 vessels) were excluded because of plaque progression from non-significant narrowings with diameter stenosis <50% at baseline or in vessels unavailable for offline QFR calculation due to poor angiographic image quality, precluding vessel contour detection or other reasons.
Statistical analysis
Analyses were performed using SAS version 9.4 (SAS Institute) or R version 3.2.4 (R Foundation for Statistical Computing). We employed standard descriptive statistical techniques. Applicable tests were 2-tailed and p<0.05 was considered statistically significant, and an interaction p-value <0.10 was considered statistically important in keeping with international suggestions16.
Survival curves were constructed for time-to-event variables with the Kaplan-Meier method and compared using the log-rank test. A mixed-effects Cox proportional hazards model contained random effects to account for multiple vessels per subject and potential variation between the 2 trials. Its fixed effects included vessel treatment (PCI or medical therapy). QFR as a continuous variable between 0 and 1 was analysed, given that an interaction term was observed in a previous study.12. The proportional hazards assumption was examined visually and via Schoenfeld residuals. Hazard ratios (HR) and their 95% confidence intervals (CI) from these Cox models demonstrate the treatment effect of PCI. For a parallel comparison, these same models were built using percentage diameter stenosis from quantitative coronary angiography instead of QFR.
Because treatment was not randomised across the QFR spectrum, we developed a propensity score to predict treatment with PCI compared to medical therapy. All clinical and procedural variables excluding QFR (since it was part of the main model) were entered into a multivariable logistic regression model to predict PCI treatment of the vessel. An additional Cox model adding this propensity score accounted for non-random treatment assignment.
Results
Figure 1 summarises the pooled cohort that included 8,044 vessels from 5,197 subjects. As depicted in Figure 1 and Figure 2, 6,013 of 8,044 (74.8%) vessels had a QFR ≤0.80, while 2,031 of 8,044 (25.2%) vessels had a QFR>0.80. A large majority of vessels with a QFR ≤0.80 underwent PCI: 5,304 of 6,013 (88.2%); however, a sizable minority of vessels with a QFR>0.80 also received PCI: 821 of 2,031 (40.4%). The sharp treatment transition around QFR=0.80 in Figure 2 reflects the FAVOR III China protocol.
Supplementary Table 3 summarises the subject characteristics for the total cohort that were broadly similar between the 2 trials. Supplementary Table 4 and Supplementary Table 5 compare subject and procedural characteristics between vessels, stratified by QFR=0.80 and also treatment (PCI or medical therapy). Broadly speaking, and as has long been appreciated for FFR, clinical and angiographic characteristics showed considerable overlap between vessels with high versus low QFR, with more severe and longer lesions producing a QFR ≤0.80, especially in the left anterior descending coronary artery.
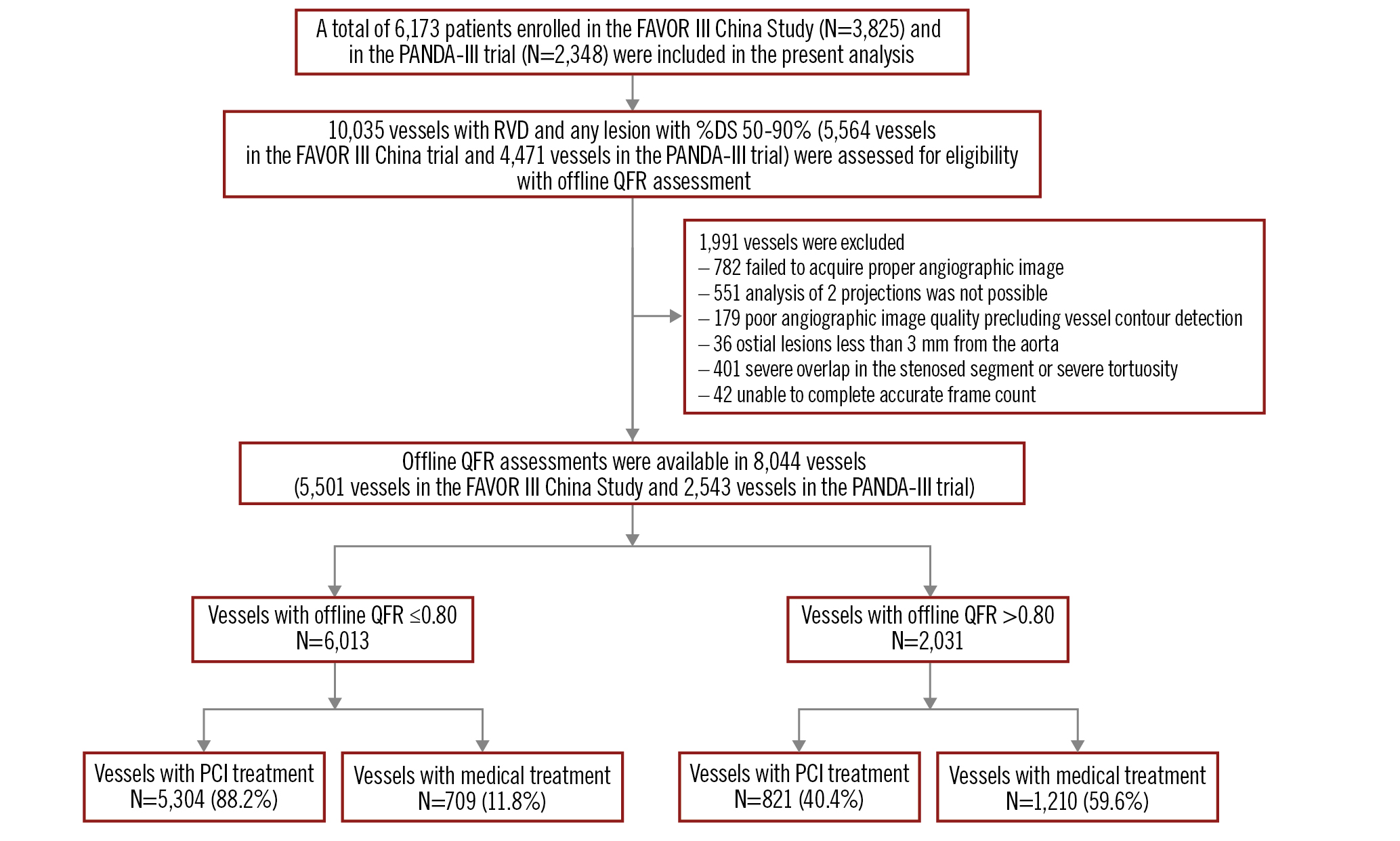
Figure 1. Study flowchart. From 2 large randomised trials, offline (centralised, post hoc, and blinded) QFR was measured by an angiographic core laboratory in all coronary arteries with any lesion of at least 50% diameter stenosis and reference vessel diameter 2.5 mm or larger. DS: diameter stenosis; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio; RVD: reference vessel diameter
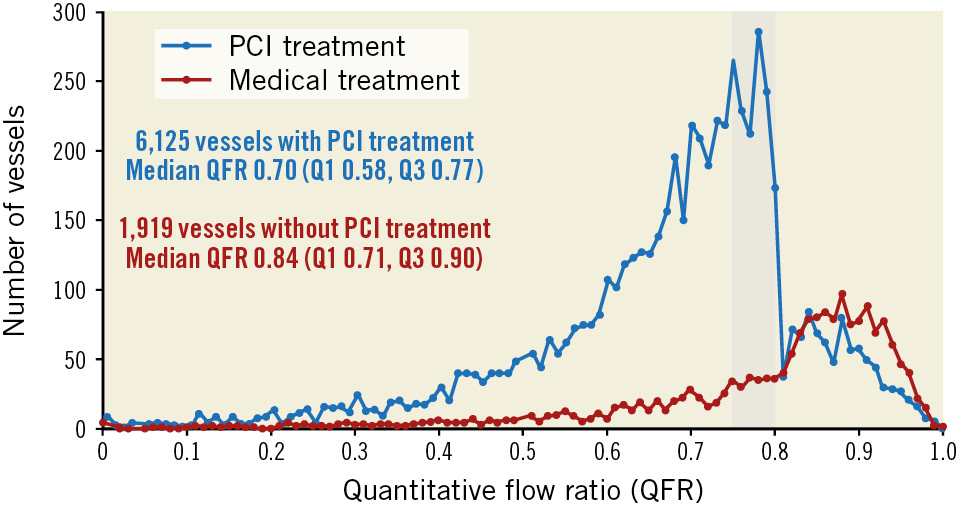
Figure 2. QFR distribution. Histogram of QFR measurements for vessels treated by medical therapy (red) or revascularisation (blue). The shaded region denotes a QFR between 0.75 and 0.80. PCI: percutaneous coronary intervention; Q1: lower quartile; Q3; upper quartile; QFR: quantitative flow ratio
Clinical outcomes
Table 1 details the number of clinical events for the primary endpoint (spontaneous MI) and secondary endpoints (cardiac death, periprocedural and total MI, and ID-TVR) in binary categories using QFR=0.80. PCI for vessels with a QFR ≤0.80 experienced MI less often than when vessels were treated medically (3.0% vs 4.6%; p=0.03). In contrast, PCI for vessels with a QFR>0.80 increased the MI risk compared with medical treatment (3.6% vs 1.2%; p<0.0001) (Figure 3).
These raw percentages using binary QFR were confirmed using continuous QFR in the Cox models summarised in Table 2. QFR showed an inverse association with spontaneous MI (HR 0.89, 95% CI: 0.79-0.99; p=0.04) that was reduced by PCI compared to medical therapy (HR 0.26, 95% CI: 0.17-0.40; p<0.0001). Additionally, the interaction term between continuous QFR and PCI treatment was modestly important (p=0.09) and numerically positive, indicating a larger treatment benefit for lesions with lower QFR values. Using a previously published interpretation12, this interaction indicated a net benefit for PCI over medical therapy to reduce total MI (spontaneous and periprocedural) beginning at QFR ≤0.64. Figure 4 and Supplementary Figure 1 display these results visually.
Only 4 cardiac deaths were attributable to a specific vessel, compared to 36 cardiac deaths without an attributable vessel. The 4 deaths all occurred in vessels with a QFR ≤0.80, whereas none occurred when the QFR>0.80. As detailed in Table 2, PCI treatment was not significantly associated with vessel-specific cardiac death, whereas continuous QFR displayed a borderline association (upper limit of confidence interval 1.01 without propensity adjustment, and 0.91 afterwards).
By definition, periprocedural MI occurred only in vessels treated with PCI, as did initial ID-TVR. While delayed ID-TVR was more common after initial medical therapy (3.2% vs 2.5%; p=0.32 when QFR>0.80, and 6.9% vs 1.9%; p<0.0001 when QFR ≤0.80), total ID-TVR (initial plus delayed) remained far more common after an initial strategy of PCI.
Supplementary Table 6 provides a parallel analysis to Table 2 but using quantitative percentage diameter stenosis instead of QFR. Unlike the results in Table 2 for QFR, percentage diameter stenosis did not show a significant association with spontaneous MI. Additionally, it showed no significant association with vessel-specific cardiac death.
Table 1. Vessel-level clinical events* during 2-year follow-up by Kaplan-Meier analysis.
| QFR ≤0.80 | QFR >0.80 | |||||
|---|---|---|---|---|---|---|
| PCI | Medical | Hazard ratio(95% CI) | PCI | Medical | Hazard ratio(95% CI) | |
| Vessels | 5,304 | 709 | 821 | 1,210 | ||
| Spontaneous MI | 0.6% (31/5,282) | 4.6% (32/705) | 0.13 (0.08-0.21) | 0.9% (7/816) | 1.2% (14/1,209) | 0.74 (0.30-1.83) |
| Periprocedural MI | 2.4% (128/5,298) | 0% (0/709) | - | 2.7% (22/821) | 0% (0/1,210) | - |
| Total MI | 3.0% (156/5,276) | 4.6% (32/705) | 0.66 (0.45-0.96) | 3.6% (29/816) | 1.2% (14/1,209) | 3.11 (1.64-5.89) |
| Cardiac death | <0.1% (2/5,260) | 0.3% (2/702) | 0.13 (0.02-0.95) | 0% (0/817) | 0% (0/1,202) | - |
| Immediate ID-TVR† | 100% (5,230/5,230) | 0% (0/705) | - | 100% (808/808) | 0% (0/1,209) | - |
| Delayed ID-TVR† | 1.9% (97/5,230) | 6.9% (48/705) | 0.26 (0.19-0.37) | 2.5% (20/808) | 3.2% (39/1,209) | 0.76 (0.44-1.30) |
| Total ID-TVR | 101.9% (5,327/5,230) | 6.9% (48/705) | - | 102.5% (828/808) | 3.2% (39/1,209) | - |
| Numeric values are represented as % (number/total). *Excluded for event analysis: no clearly identifiable culprit vessel for 25 non-procedural MI events (25 patients with 32 vessels), 3 periprocedural MIs (3 patients with 6 vessels), and 36 cardiac deaths; additionally, 78 revascularisations (78 patients with 92 vessels) in vessels without ≥50% diameter stenosis at baseline and thus initially ineligible for QFR analysis or in vessels without QFR calculation. †Immediate = as part of the initial procedure, delayed = either a first PCI of a medically treated vessel or a repeat PCI of an initially revascularised vessel, total = immediate + delayed. CI: confidence interval; ID-TVR: ischaemia driven target vessel revascularisation; MI: myocardial infarction; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio | ||||||
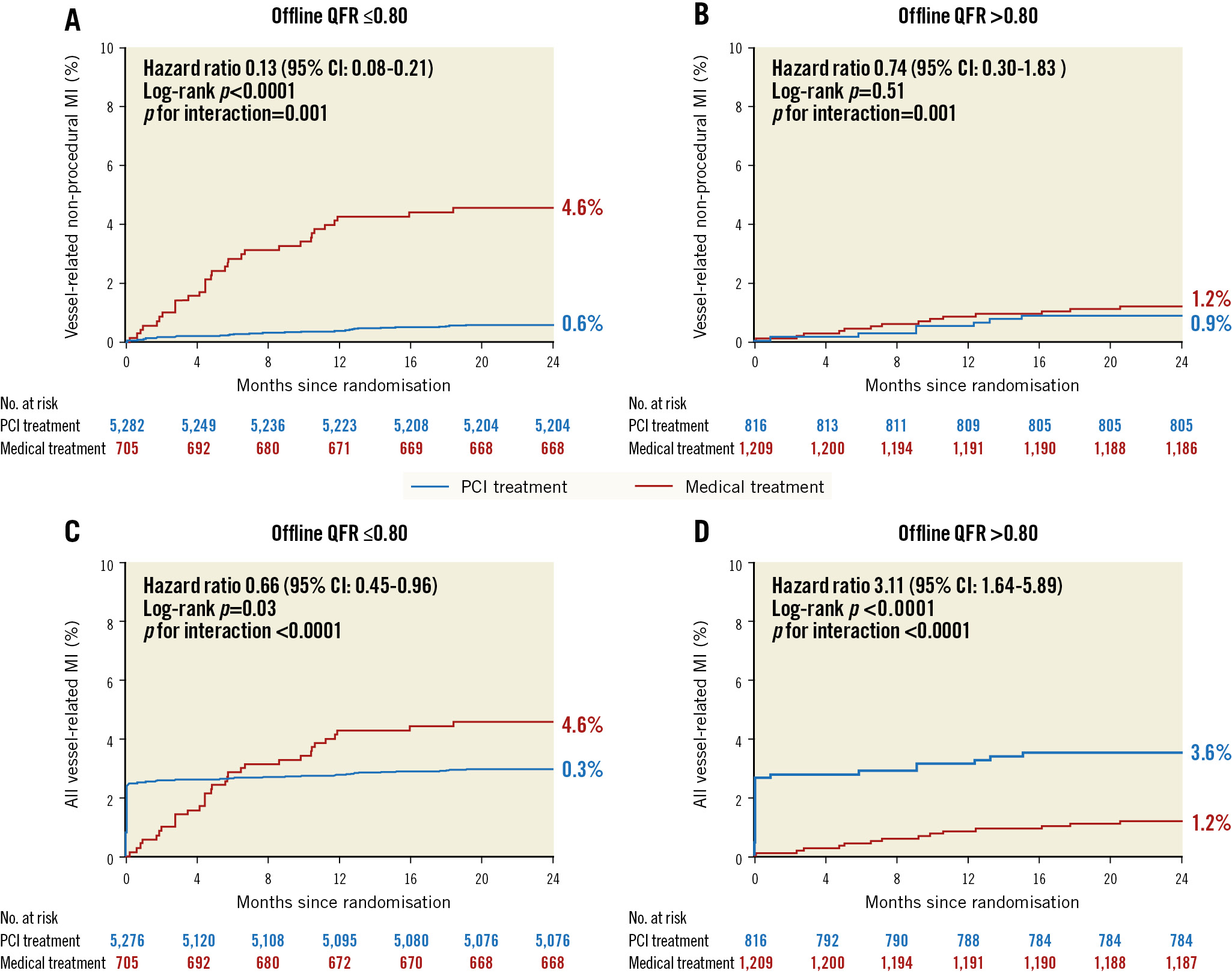
Figure 3. Kaplan-Meier survival curves for myocardial infarction when using a QFR of 0.80 as the cut-off value. Vessel-level spontaneous myocardial infarction (A, B) and all myocardial infarction (C, D) categorised by QFR (high vs low) and treatment (medical therapy or revascularisation). CI: confidence interval; MI: myocardial infarction; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio
Table 2. Cox proportional hazards models for 2-year endpoints
| Spontaneous MI hazard ratio(95% CI) | Cardiac death hazard ratio (95% CI) |
|
|---|---|---|
| Continuous QFR* | 0.89 (0.79-0.99) | 0.66 (0.43-1.01) |
| PCI treatment† | 0.26 (0.17-0.40) | 0.31 (0.04-2.22) |
| Propensity score adjustment‡ | ||
| Continuous QFR | 0.84 (0.72-0.97) | 0.58 (0.37-0.91) |
| PCI treatment | 0.29 (0.17-0.48) | 0.28 (0.03-2.48) |
| Numeric values are hazard ratios (95% CI).*Hazard ratio per 0.05 increase in QFR. †Compared to medical therapy. ‡Variables included in propensity score model were vessel location, bifurcation lesion, severe tortuosity, severe calcification, tandem lesion, reference vessel diameter, minimal lumen diameter, diameter stenosis, and total lesion length per vessel. CI: confidence interval; MI: myocardial infarction; QFR: quantitative flow ratio; PCI: percutaneous coronary intervention | ||
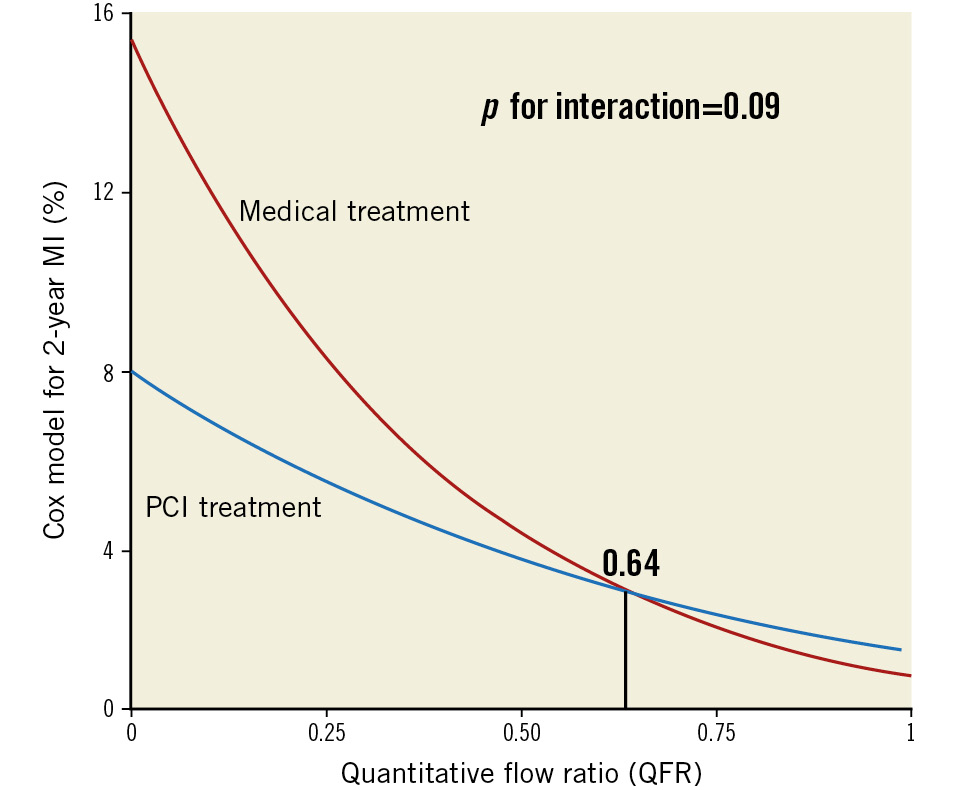
Figure 4. Continuum of QFR risk and benefit. After 2 years, vessels with lower QFR values experience a higher rate of myocardial infarction (MI) when treated medically. Percutaneous coronary revascularisation (PCI) reduces this risk most at lower values and, due to periprocedural MI, increases risk for higher values with an outcome-based threshold of 0.64. QFR: quantitative flow ratio
Treatment propensity score
Supplementary Table 7 provides the coefficients for each parameter used to build a model to predict PCI treatment. In general, baseline vessel and lesion characteristics including vessel location, bifurcation lesion, severe calcification, tandem lesion, reference vessel diameter, minimal lumen diameter, diameter stenosis, and total lesion length per vessel showed significant differences between vessels with and without PCI treatment. Adjusting for this propensity score did not materially alter the earlier results, as detailed in Table 2.
Discussion
A continuous, inverse relationship existed in our study between the QFR value of a vessel and its subsequent risk for total MI (spontaneous plus periprocedural). PCI, compared to medical therapy, reduced this risk beginning at a QFR value of 0.64. Because of the interaction between the QFR value and the magnitude of PCI benefit, our findings indicate that vessels with lower QFR values received a proportionally larger reduction in their risk of MI (Central illustration). This same continuum was not observed for quantitative percentage diameter stenosis, indicating a unique advantage offered by QFR over a simplistic anatomical metric.
We did not find that PCI significantly reduced the risk of vessel-related cardiac death, which was a rare event. Despite including over 8,000 vessels followed for 2 years, we could identify only 4 cardiac deaths arising from a specific coronary artery, outnumbered by the more numerous 36 cardiac deaths that occurred without a definitive relationship to an epicardial territory. These results support prior randomised trials that have also shown no benefit of increased revascularisation on cardiac death123 as well as cross-sectional studies showing no relationship between rates of MI and cardiac death across trials17.
It is tempting to focus solely on delayed target vessel revascularisation (either first PCI of a medically treated vessel or repeat PCI of an initially revascularised vessel), which is modestly reduced by a strategy of initial PCI but relatively uncommon (pooled incidence <3% over 2 years). However, Table 1 makes it clear that the rate of total ID-TVR is over 20-fold larger when accounting for both initial and delayed PCI. Additionally, 77 vessels in 66 subjects received delayed ID-TVR as a result of disease progression, despite having no initial lesion with a diameter stenosis 50%, and thus were ineligible for baseline QFR analysis and would not have been considered for PCI. The 77 delayed ID-TVR were as numerous as the 87 delayed ID-TVR in vessels treated medically after baseline QFR assessment.
Therefore, an initial strategy of PCI leads to more ID-TVR and more periprocedural MI, essentially by definition. However, initial PCI can be associated with 2 clinical advantages when applied appropriately. First, vessels with a QFR ≤0.64 experience fewer total MI after PCI, especially as the QFR value falls. Second, PCI improves angina for patients with symptoms. Although not a metric collected by either FAVOR III China or PANDA-III, freedom from angina was more prevalent after PCI in COURAGE18, ORBITA19, and ISCHEMIA20, especially when considering only the subset with frequent angina.
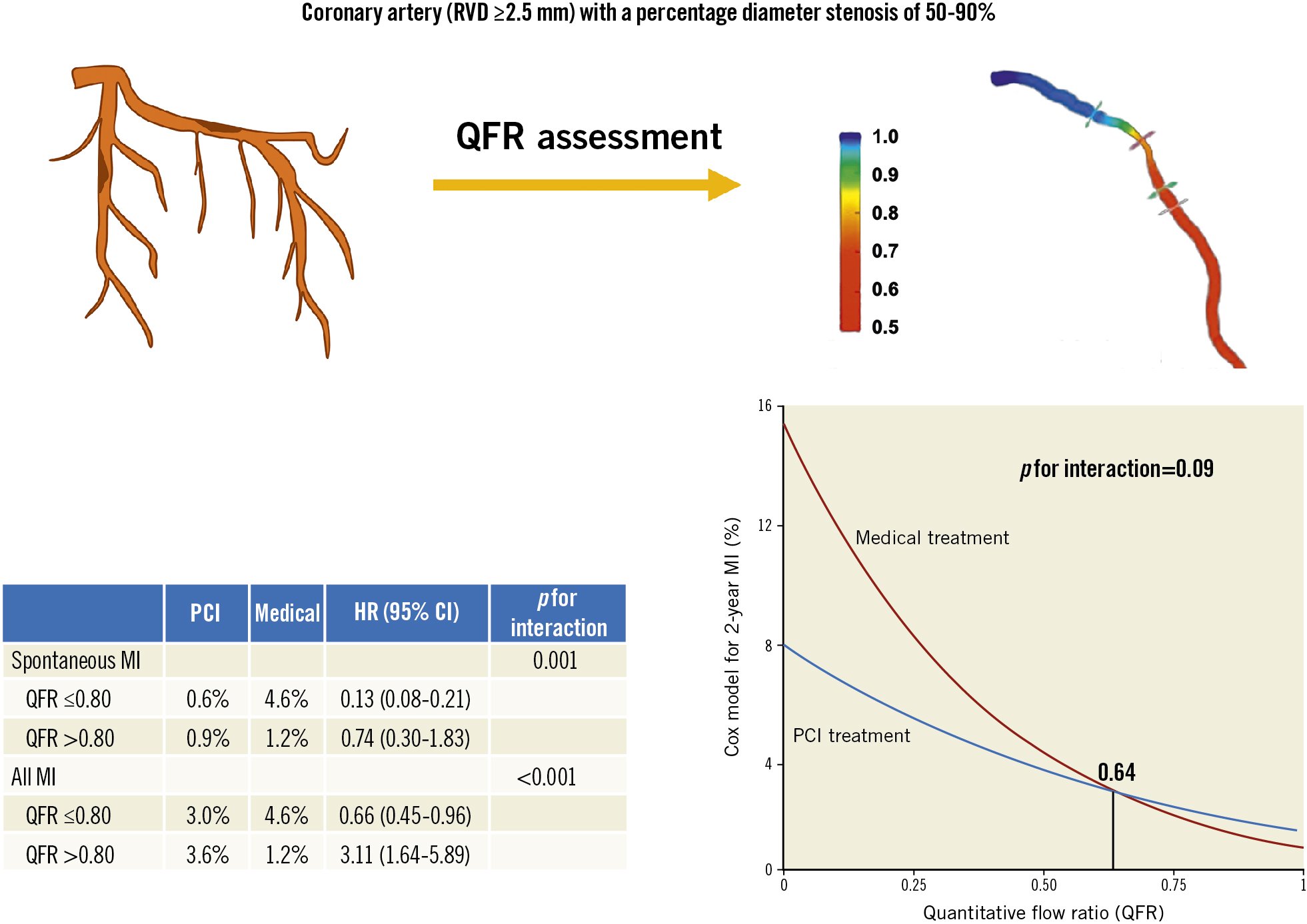
Central illustration. A continuous, inverse relationship exists between the QFR value of a vessel and its subsequent risk for MI, with a net benefit for PCI over medical therapy to reduce MI beginning at QFR=0.64. CI: confidence interval; HR: hazard ratio; MI: myocardial infarction; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio; RVD: reference vessel diameter
Comparison to existing literature
To our knowledge, no prior study has examined the relationship between continuous QFR and subsequent clinical events as modified by PCI. However, a substantial parallel literature exists for FFR122122, which largely shows similar inverse associations and comparable threshold values of 0.6422 and 0.6712 that support the 0.64 displayed in Figure 4. Supplementary Table 8 compares our results against 5 prior RCT using different selection criteria for revascularisation. All studies show a reduction in spontaneous MI, reinforcing the reproducibility of this finding. Potentially, additional information such as plaque composition, pressure gradients along the vessel, and biomarkers may further augment the absolute and relative benefits to PCI already seen (Supplementary Table 7), using primarily functional, hyperaemic tools like FFR, QFR, and non-invasive stress testing. Parallel working on the ischaemic continuum of risk using FFR12 supports our findings using QFR. Both bodies of work use post hoc or observational designs, with logistical tradeoffs between FFR (cost of invasive wire but superior precision) and QFR (can be applied retrospectively to any angiogram after initial equipment and software acquisition).
Limitations
In addition to the intrinsic limitations of any post hoc observational design that was not prespecified, our study excluded 1,991 of 10,035 (19.8%) vessels whose QFR could not be computed, as reported in Figure 1. Treatment assignment was not randomised on a per-vessel basis due to both the underlying RCT protocols and operator compliance, leading to the uneven distribution seen in Figure 2. Our propensity score may not have fully neutralised the selection bias of patients and vessels for a specific treatment. As a retrospective study, detailed medication information was not collected. Some events could not be definitively attributed to a specific vessel, due to lack of repeat angiography or clinical ambiguity. Angina severity was not quantified before or after treatment, with either physician or patient assessments. In the present study, follow-up extended for only 2 years. Therefore the 3- to 5-year outcomes presented in Supplementary Table 8 need further study. These limitations could be overcome through a dedicated randomised trial whereby lesions along the QFR continuum receive either PCI or medical therapy. However, we believe that such a trial poses significant practical limitations given results from the studies in Table 1 already showing a reduction in spontaneous MI from revascularisation. As such, the strength of the current analysis comes from its insights into the QFR continuum via existing randomised trials.
Conclusions
Vessels with a reduced QFR experienced a higher rate of spontaneous MI when treated medically instead of with PCI. A continuous, inverse relationship between the QFR value of a vessel and its subsequent risk for MI was demonstrated, providing physicians with an angiographic tool for optimising vessel selection for PCI.
Impact on daily practice
The quantitative flow ratio (QFR) identifies functionally ischaemic lesions that may benefit more from percutaneous coronary intervention (PCI) than medical therapy. Vessels with lower QFR values receive a proportionally larger reduction in their risk of MI when treated with PCI instead of medical therapy. A continuous, inverse relationship exists between the QFR value of a vessel and its subsequent risk for myocardial infarction (MI), with a net benefit for PCI over medical therapy to reduce MI beginning at QFR ≤0.64. Further research is needed to refine the role of QFR in guiding vessel revascularisation decisions.
Funding
The study was supported by research grants from the National High Level Hospital Clinical Research Funding (2022-GSP-QN-6 and 2022-GSP-GG-20), the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences (NCRC2020001), and the Beijing Municipal Science and Technology Commission (Z191100006619107).
Conflict of interest statement
N.P. Johnson received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis; has received significant institutional research support from Philips/Volcano Corporation (DEFINE-FLOW, NCT02328820) and CoreAalst (PPG Global, NCT04789317) for unrelated studies of coronary physiology; and has pending patents on diagnostic methods for quantifying aortic stenosis and TAVI physiology and on algorithms to correct pressure tracings from fluid-filled catheters. S. Tu is the cofounder of Pulse Medical and reports grants and consultancy from Pulse Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

