In this issue of EuroIntervention, a fascinating substudy by Zhang et al1 entitled “Outcomes of quantitative flow ratio-based percutaneous coronary intervention in an all-comers study” on the quantitative flow ratio (QFR) in PANDA III (ClinicalTrials.gov: NCT02017275) offers enduring lessons regarding diagnostic trial design and analysis2.
Focus on disagreements
Trials comparing diagnostic tests or strategies differ fundamentally from device or drug studies. Stated simply, a patient can undergo two different tests but cannot receive two different treatments. Most trials explicitly link test results to therapy; for example, QFR >0.8 receives medical treatment whereas QFR ≤0.8 undergoes percutaneous coronary intervention (PCI). Consequently, such results dictate treatment directly. As has been astutely observed2, comparing two tests leading deterministically to treatment must focus on disagreements by performing both tests in every patient. Only the subgroup of patients with divergent decisions (for example, angiographically significant yet QFR >0.8) can lead to differential outcomes by way of distinct therapies (PCI versus medical therapy). Identical decisions produce identical outcomes via identical treatment (to continue the example, both angiographically significant and QFR ≤0.8 receive PCI).
Modifiable, causal endpoints
Intracoronary physiology like QFR or fractional flow reserve (FFR) provides a vessel-level diagnosis. Treatment with PCI also remains vessel specific. Furthermore, PCI and medical therapy have repeatedly shown no differences in all-cause or cardiovascular mortality when applied to stable lesions. Therefore, we should focus our attention on target vessel outcomes like myocardial infarction (TVMI) and revascularisation (TVR). Including non-modifiable endpoints like mortality, or non-causal events from non-target vessels, adds an equal number of events to both groups, inflating rates but without altering their difference. Finally, spontaneous and periprocedural TVMI must be separated and probably only the former emphasised given the vagaries of the latter.
Blinded QFR in PANDA III
With this important background, we can turn our attention to the current substudy1 that measured QFR post hoc from angiograms collected as part of a randomised trial of two different stent platforms3. Because QFR was not calculated at the time of decision-making, this design mimics blinding but at the cost of excluding 40% of angiograms, mainly due to acquisition or anatomic issues. Additionally, 31% of patients in PANDA III presented with MI3, split roughly equally between those with and without ST-segment elevation. Thus, some of the lesions included in this QFR substudy1 appear to have been infarct culprits – vessels not recommended for physiologic interrogation. Additionally, the definition of “ischaemia-driven” revascularisation remained somewhat circular, since it could include no symptoms or functional testing but only diameter stenosis ≥70% as already present at baseline in about half of untreated QFR ≤0.8 vessels.
In Figure 1, we provide a visual critique of the substudy analysis1 that mixed QFR and treatment subgroups, included non-modifiable endpoints like mortality, and blurred target and non-target vessel events. Instead of comparing the “rows” as presented in Figure 1, a more insightful analysis would have compared its “columns” using endpoints of TVR and spontaneous TVMI. The distinction becomes immediately clear by noting that each row contains some mixture of positive and negative QFR lesions treated both medically and with PCI. Only analysing the same QFR category (abnormal or normal) with contrasting treatments (medical versus PCI) provides an answer about the penalty of not following a physiology-derived recommendation.
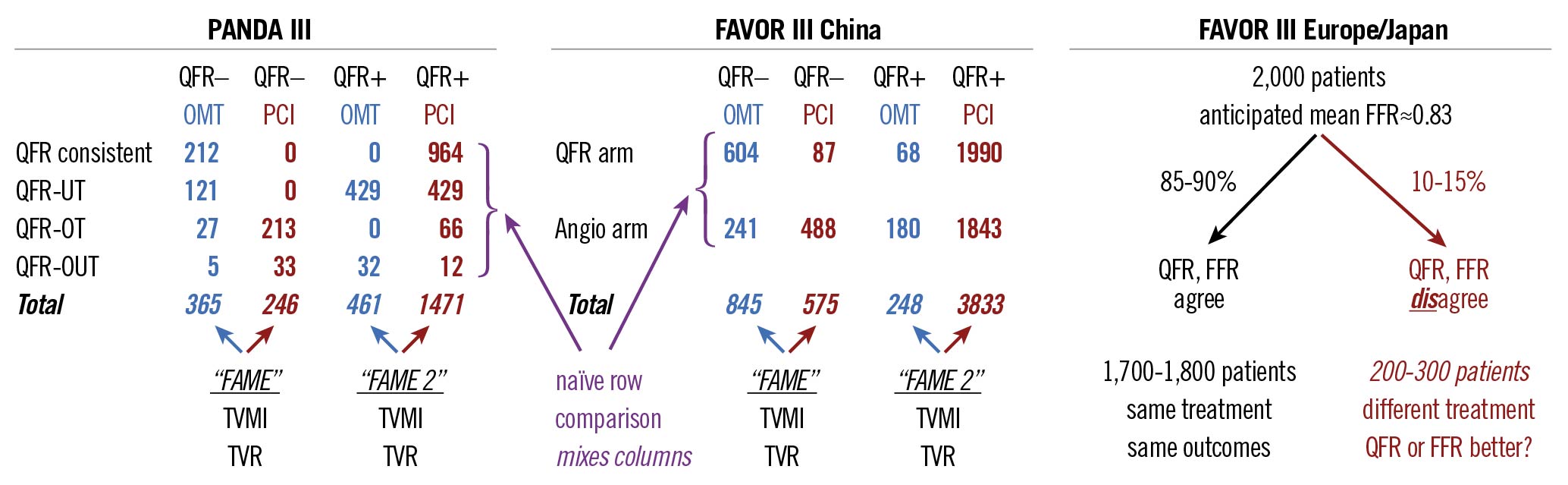
Figure 1. Visual critique of three trials using the quantitative flow ratio (QFR). angio: angiography; FAME, ClinicalTrials.gov: NCT00267774; FAME 2, ClinicalTrials.gov: NCT01132495; FAVOR III China: cited4; FAVOR III Europe/Japan, ClinicalTrials.gov: NCT03729739; FFR: fractional flow reserve; OMT: optimal medical therapy; PANDA III: cited13; PCI: percutaneous coronary intervention; QFR-OT: QFR-based overtreatment; QFR-OUT: QFR-based over- and undertreatment; QFR-UT: QFR-based undertreatment; TVMI: target-vessel myocardial infarction; TVR: target-vessel revascularisation
Despite this confusing heterogeneity, we can nevertheless observe several themes in the current analysis1. First, their Figure 3B confirms that all-cause mortality remains rare and similar among QFR and treatment groups. Second, their Figure 3C shows an abrupt rise of periprocedural infarctions with far fewer spontaneous events (roughly a 4:1 ratio as per their Table 2) over the next two years. Third, their Figure 3D demonstrates the expected, steady accumulation of further PCI – lowest for the “QFR consistent” row and highest for the “QFR-based undertreatment” row. These results recapitulate familiar themes: FFR-guided PCI does not affect mortality; FFR-negative lesions do well with medical therapy; FFR-positive lesions frequently cross-over to PCI after initial medical therapy; and the ongoing controversy regarding periprocedural versus spontaneous MI.
Test twice, treat once
What are the implications of the current substudy1 for other angiography-derived FFR trials? The recent FAVOR III China study randomised patients to angiographic-based versus QFR-guided PCI4. As visually critiqued in our Figure 1, its “row” design suffered from all the same flaws as the PANDA III substudy1. We strongly urge its investigators to perform a “column” analysis that is likely to show an even greater impact of QFR on modifiable, causal outcomes (hinted at in Figure 3 of the FAVOR III report4 by the 0.41 hazard ratio for QFR >0.85 versus the composite 0.65 for the entire cohort that has been diluted by the large number of vessels with QFR <0.8 treated exactly the same, regardless of randomisation).
The ongoing FAVOR III Europe/Japan trial (ClinicalTrials.gov: NCT03729739) is randomising 2,000 patients to either QFR-guided or FFR-guided PCI using a non-inferiority design. As visually critiqued in Figure 1, its meaningful (discordant treatment) sample size falls to 200-300 patients given the anticipated 85-90% binary agreement for mild lesions (average FFR 0.83) that often undergo physiologic assessment in clinical practice5, although a different distribution might arise in a PCI trial (FAVOR III China4 had mean QFR 0.72). While unfortunately too late to address this incorrect design, QFR can be measured post hoc from the angiograms in the FFR arm to understand the level of discordance in the trial population and perform best- and worst-case estimates of clinical outcomes among discordant patients.
In summary, trials of diagnostic tests require a different design than the rote “A versus B” used when studying a drug or device. Our Figure 1 details how biased estimates of test impact can arise from a naïve analysis of an improperly designed trial, and how to overcome this tendency by refocusing on discordances.
Conflict of interest statement
N. P. Johnson received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis; received significant institutional research support from St. Jude Medical (CONTRAST, NCT02184117) and Philips/Volcano Corporation (DEFINE-FLOW, NCT02328820) for studies using intracoronary pressure and flow sensors; has an institutional licensing agreement with Boston Scientific for the smart-minimum FFR algorithm commercialised under 510(k) K191008; and has pending patents on diagnostic methods for quantifying aortic stenosis and TAVI physiology, and also algorithms to correct pressure tracings from fluid-filled catheters. C. Collet received research grants from Biosensors, HeartFlow, and Abbott Vascular; and has received consultancy fees from HeartFlow, Abbott Vascular, Boston Scientific, Opsens, and Philips/Volcano.
Supplementary data
To read the full content of this article, please download the PDF.

