The Multivessel TALENT trial is an ongoing randomised controlled trial comparing 2 drug-eluting stents in patients with de novo three-vessel disease without left main disease1. The study design mandates an offline assessment of quantitative flow ratio (QFR) pre-percutaneous coronary intervention (PCI) to prospectively identify which lesions should be treated. The rationale of this study lies in the post hoc analysis of the SYNTAX II trial, which established that a post-PCI QFR ≥0.91 was the optimal cut-off for a lower risk of 2-year vessel-oriented composite endpoints (VOCE). ClinicalTrials.gov: NCT04390672.
The current study was a vessel-based analysis using the post-PCI QFR from the first 775 patients randomised in the Multivessel TALENT study and was conducted before event adjudication commenced to forecast the rate of VOCE at 2 years for the entire population.
The details of the Multivessel TALENT trial design have been previously published1. The powered secondary endpoint is a superiority comparison in the per-protocol analysis of VOCE, a composite of vessel-related cardiovascular death, vessel-related myocardial infarction, and clinically and physiologically indicated target vessel revascularisation at 24 months post-procedure1.
QFR is an angiography-derived fractional flow reserve (FFR) technology which was analysed offline by either QAngio XA 3D QFR imaging software (Medis Medical) or AngioPlus Core software (Pulse Medical) with Murray law-based QFR analysis (μQFR), depending on whether there were one or two angiographic views available (Supplementary Figure 1). Both systems have equivalent diagnostic performance123. Details of the analysis are described in Supplementary Appendix 1.
Based on previous reports describing the impact of post-PCI QFR values on 2-year VOCE2, a favourable VOCE rate is expected when the post-PCI vessel QFR is ≥0.91.
To predict 2-year VOCE in the current study based on the QFR measurements available in this interim sample, we first fitted a logistic regression model to the SYNTAX II trial data, with 2-year VOCE as the binary response variable and post-procedure QFR as the continuous predictor variable. A restricted cubic spline was used to model the change in the log-odds of 2-year VOCE, with changes in QFR to flexibly model any non-linearity. The 95% prediction interval (PI) for the 2-year VOCE rate was obtained using the predicted probabilities in the interim sample and bootstrap resampling. The details are presented in Supplementary Appendix 1.
This study included the first 775 consecutive patients randomised in the Multivessel TALENT trial. A total of 1,674 lesions were treated between September 2020 and February 2023. The mean number of vessels treated per patient was 2.32.
Baseline patient and lesion characteristics are presented in Supplementary Table 1 and Supplementary Table 2, with comparisons to the SYNTAX II trial.
Analysable QFRs were available in 1,557 vessels pre-PCI and 1,403 vessels post-PCI (Supplementary Figure 2). Details of non-analysability are presented in Supplementary Appendix 1.
Supplementary Figure 3 shows pre- and post-PCI QFRs presented as a cumulative distribution curve. Of note, 146 out of 1,557 vessels (9%) were treated even though the pre-PCI QFR was>0.8. A post-PCI QFR ≥0.91 was achieved in 1,072 of the 1,403 (76%) treated vessels.
Based on predictions from the fitted SYNTAX II model and the available post-procedure QFRs in the interim Multivessel TALENT data, the predicted rate of 2-year VOCE in the trial was 6.1%, with a bootstrap 95% PI of 4.8% to 7.4% (Supplementary Table 3, Supplementary Figure 4, Supplementary Figure 5, Supplementary Figure 6). The Central illustration summarises the scheme and the key results of this study.
The current analysis has been conducted on the first half of the planned study population in an attempt to ascertain (1) whether appropriate vessels are being selected for revascularisation and (2) whether PCI is being performed optimally. Its other main purpose was to reassure the steering committee and data and safety monitoring board on the validity of the statistical sample size assumptions.
A SYNTAX II substudy has established that a post-PCI QFR ≥0.91 significantly improves 2-year VOCE2. The current study has a similar number of vessels treated per patient to that seen in SYNTAX II. Given that 76% of vessels achieved the optimal threshold, we can reasonably assume that the 2-year VOCE of the Multivessel TALENT population could be 6.1%, with an acceptable PI.
QFR identifies stenotic vessels with a pressure-derived FFR ≤0.80 with good diagnostic accuracy, without need for a pressure wire or induction of pharmacological hyperaemia. Recently, FAVOR III China demonstrated significantly fewer myocardial infarctions and ischaemia-driven revascularisations in one- and two-year major adverse cardiac events in the QFR versus the angiography-guided group45.
For 24% of the lesions, single-view software was used, so post-PCI QFR analyses were ultimately analysable in 84% of the cohort.
The stated assumptions that (1) the available QFR data of these initial patients will be representative of the eventual full trial sample and that (2) the relationship between QFR and VOCE in Multivessel TALENT is similar to SYNTAX II, whilst plausible, can only be verified upon completion of the study.
Favourable post-PCI QFRs were obtained in 76% of patients, resulting in an expected overall favourable VOCE rate of 6.1%, with a boundary of 4.8% to 7.4%.
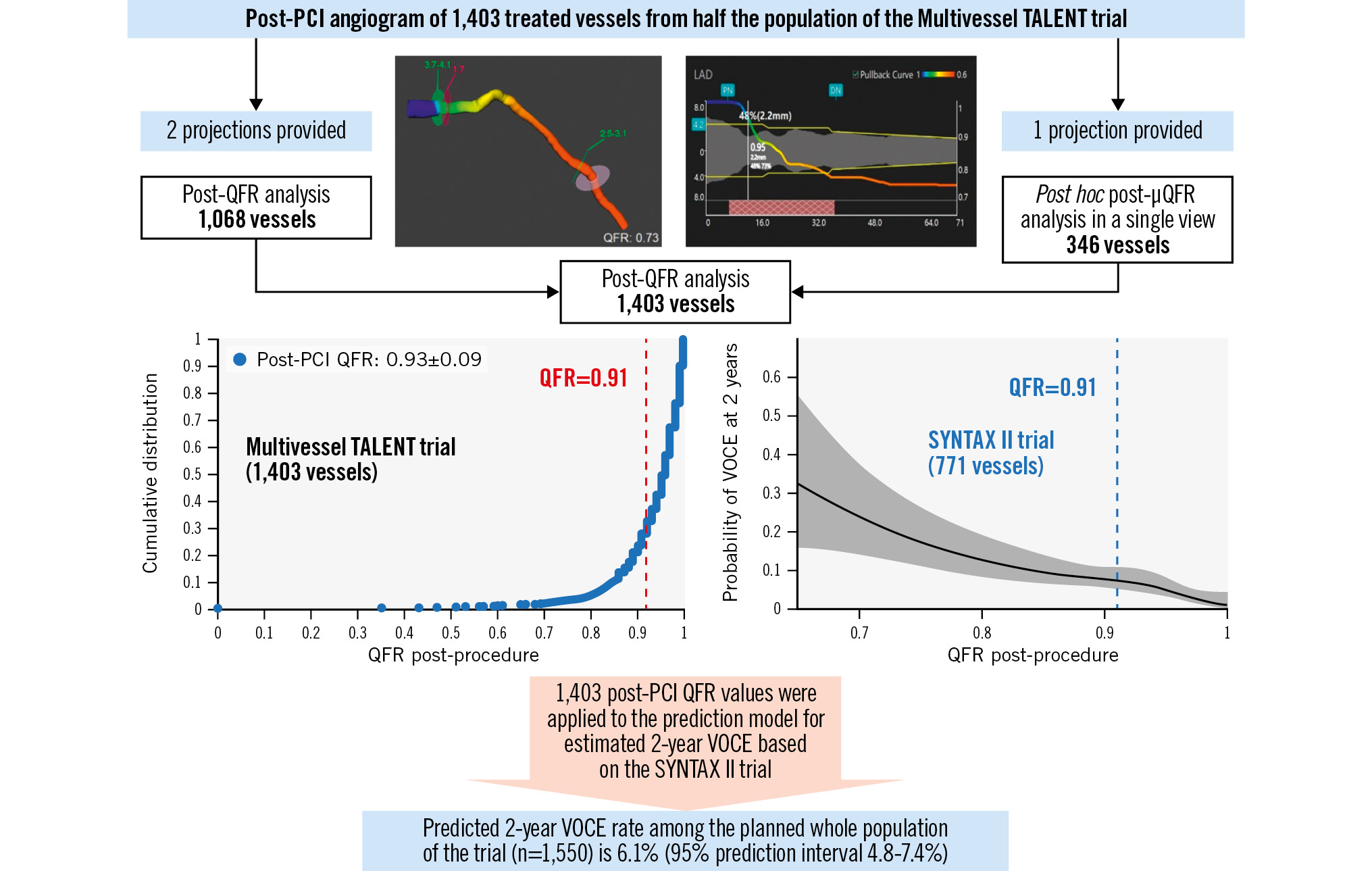
Central illustration. Prediction of 2-year VOCE for the whole population of the ongoing Multivessel TALENT trial based on post-PCI QFR results of half of the population. Two different offline angiography-derived FFR software were used, depending on whether there were one or two angiographic views available, in order to obtain the pre- and post-PCI QFR in the maximum number of participants in the ongoing Multivessel TALENT trial before event adjudication. In cases where only baseline or postprocedural QFR were available, µQFR analysis was added, pre- or post-PCI, in the same vessel. Eleven vessels were analysed with both techniques post-procedure. An optimal post-PCI QFR was obtained in 76% of the treated vessels. Based on the relationship between post-PCI QFR and 2-year VOCE in the SYNTAX II trial, the predicted 2-year VOCE in the entire Multivessel TALENT trial population would be 6.1%, with a boundary of 4.8% to 7.4%. FFR: fractional flow reserve; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio; VOCE: vessel-oriented composite endpoint; µQFR: Murray law-based QFR
Funding statement
The Multivessel TALENT trial is an investigator-initiated trial sponsored by the University of Galway, which received an unrestricted grant from SMT (Sahajanand Medical Technologies, India) to investigate the value of QFR in multivessel disease.
Conflict of interest statement
K. Ninomiya reports a grant from Abbott Medical Japan outside the submitted work. S. Tu reports receiving research grants and consultancy fees from Pulse Medical. M. Sabaté has received consultancy fees from Abbott and iVascular, outside the submitted work. H. Möllmann reports speaker honoraria from Abbott, Boston Scientific, and SMT. P. Serruys reports personal consultation fees from Philips/Volcano, SMT, Novartis, Xeltis, and Meril Life, outside the submitted work. J. Reiber is Chief Scientific Officer at Medis Medical Imaging Systems. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

