Abstract
Aims: The purpose of the Multivessel TALENT trial is to compare clinical outcomes of the novel Supraflex Cruz stent with those of the SYNERGY stent in patients with three-vessel disease (3VD) undergoing state-of-the-art percutaneous coronary intervention (PCI).
Methods and results: In this prospective, randomised, 1:1 balanced, multicentre, open-label trial, 1,550 patients with de novo 3VD without left main disease will be assigned to the Supraflex Cruz or SYNERGY arm. The following treatment principles of “best practice” PCI will be applied: Heart Team consensus based on SYNTAX score II treatment recommendation, functional lesion evaluation by quantitative flow ratio (QFR), stent optimisation by intravascular imaging, optimal pharmacological treatment and prasugrel monotherapy. The primary endpoint is a non-inferiority comparison of the patient-oriented composite endpoint (POCE) of all-cause death, any stroke, any myocardial infarction, or any revascularisation, at 12 months post procedure. The powered secondary endpoint is a superiority comparison of the vessel-oriented composite endpoint (VOCE), defined as vessel-related cardiovascular death, vessel-related myocardial infarction, or clinically and physiologically indicated target vessel revascularisation, at 24 months.
Conclusions: The Multivessel TALENT trial will be evaluating a novel treatment strategy for complex coronary artery disease with state-of-the-art PCI based on angiography-derived QFR with novel ultra-thin Supraflex Cruz stents, compared with SYNERGY stents. Clinical Trial Registration URL: https://www.clinicaltrials.gov/ct2/show/NCT04390672. Unique Identifier: NCT04390672
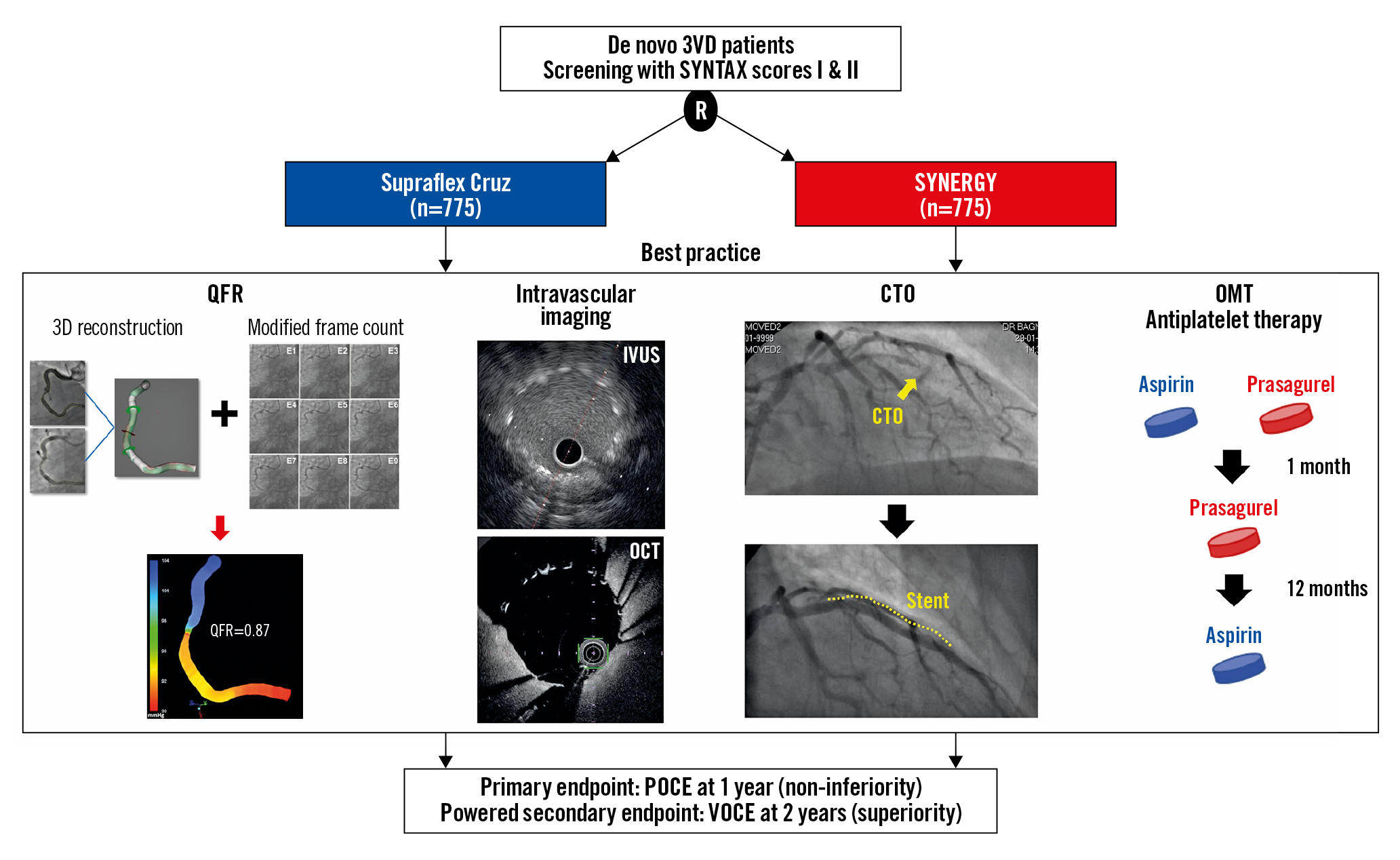
Visual summary. Flow chart of the Multivessel TALENT trial. 3VD: three-vessel disease; QFR: quantitative flow ratio; IVUS: intravascular ultrasound; OCT: optical coherence tomography; CTO: chronic total occlusion; OMT: optimal medical therapy; POCE: patient-oriented composite endpoint; VOCE: vessel-oriented composite endpoint
Introduction
The all-comers TALENT trial1 demonstrated non-inferiority of the biodegradable polymer-coated and ultra-thin strut sirolimus-eluting Supraflex™ stent (Sahajanand Medical Technologies, Mumbai, India) compared to the durable polymer-coated everolimus-eluting XIENCE stent (Abbott Vascular, Santa Clara, CA, USA) in terms of occurrence of the device-oriented composite endpoint (DOCE) at one year (cardiac death, target vessel myocardial infarction [MI], or clinically indicated target lesion revascularisation [TLR]) (4.9% in the Supraflex arm vs 5.3% in the XIENCE arm, pnon-inferiority<0.0001). In the per-protocol analysis, a 61% relative reduction of clinically indicated TLR was found in the Supraflex arm compared to the XIENCE arm (1.2% in the Supraflex arm vs 3.1% in the XIENCE arm, p=0.021). This result was corroborated by the fact that thin-strut drug-eluting stents (DES) decrease acute thrombogenicity and promote faster endothelialisation, compared with thick-strut DES2,3. The Supraflex Cruz stent is basically similar in many respects to the Supraflex stent (a biodegradable polymer-coated, ultra-thin strut sirolimus-eluting stent, with the same density of cytostatic drug), but has long dual Z connectors from “valley to valley” between the strut rings, instead of short S-links connecting “peak to peak” of the strut rings. The new mechanical platform increases the flexibility, trackability and pushability of the stent4. In addition, the Supraflex Cruz uses a softer balloon for stent retention and retrieval post deployment. Furthermore, the proximal shaft of the balloon was redesigned to improve the crossability of the device. These are essential assets in the treatment of complex coronary artery disease. Therefore, we designed a new randomised controlled trial with the novel Supraflex Cruz stent in patients with de novo three-vessel disease (3VD) without left main disease.
The recent SYNTAX II trial in patients with 3VD5 applied five treatment principles described as “best practice” in the field of percutaneous coronary intervention (PCI)6 (Supplementary Table 1): (i) patient selection based on SYNTAX score II7 recommendation and assessment of equipoise mortality with surgical treatment by Heart Team consensus5; (ii) physiological assessment of stenotic lesions by pressure-derived instantaneous wave-free ratio (iFR) or fractional flow reserve (FFR) and treatment targeting only the functionally significant lesions8; (iii) post-stent optimisation by intravascular imaging9; (iv) PCI of chronic total occlusion (CTO) performed by locally accredited experts in CTO10; and (v) optimal medical treatment before, during and after PCI11.
Physiological assessment, iFR/FFR for all vessels can be time-consuming, expensive and cumbersome. Therefore, the designers of the trial replaced the pressure wire-derived physiological assessment with an angiography-derived physiological assessment, quantitative flow ratio (QFR)12. In addition, novel antiplatelet therapy strategies, such as short-duration dual antiplatelet therapy (DAPT) and P2Y12 inhibitor monotherapy, have recently shown safety and superior efficacy in patients with multivessel disease13 when compared to conventional DAPT. Therefore, DAPT with aspirin and prasugrel for one month, followed by 11 months of prasugrel monotherapy, will be implemented and followed by aspirin monotherapy at one year.
Methods
STUDY DESIGN
The Multivessel TALENT study (ClinicalTrials.gov, NCT04390672) is a prospective, randomised, 1:1 balanced, controlled, multicentre, open-label study comparing clinical outcomes between the Supraflex Cruz and SYNERGY™ (Boston Scientific, Marlborough, MA, USA) stents. Approximately 60 sites in Europe will participate (Supplementary Table 2). Randomisation will be performed via web-based software, and will be stratified by centre and blocked, with randomly permuted block sizes of two and four, after written consent is obtained and QFR is analysed by a blinded core lab (Supplementary Figure 1).
Patients with de novo 3VD without left main disease will be treated according to state-of-the-art PCI after selection based on SYNTAX score II treatment recommendations (i.e., PCI only or equipoise coronary artery bypass grafting [CABG]/PCI) and Heart Team discussion5; functional evaluation for stenotic lesion (ESC guidelines [GL], I,A)8 by QFR; intravascular ultrasound (IVUS)/optical coherence tomography (OCT) optimisation (ESC GL, IIa,B)9; contemporary CTO techniques10 (if applicable); and optimal medical therapy11.
Patients will be followed up for two years after the index procedure and contacted at 30 days, 6 months, 12 months and 24 months. The informed consent form (ICF) will also contain a provisional agreement for a five-year follow-up. This five-year follow-up will be performed at the sole discretion of the chief investigator, sponsor and grant giver, if funding is available. All clinical events will be adjudicated by an independent clinical events committee (CEC). Serious adverse events will be periodically reviewed by an independent data safety and monitoring board (DSMB). Details on the composition, roles, and responsibilities of the Coordinating Centre/Academic Research Organisation, Steering Committee, CEC, and DSMB are described in Supplementary Appendix 1. The data management plan and quality control are presented in Supplementary Appendix 2 and Supplementary Appendix 3.
PATIENT POPULATION
A total of 1,550 patients with de novo 3VD without left main disease, for whom PCI only or equipoise CABG/PCI has been recommended according to the SYNTAX score II and local Heart Team consensus, will be randomised in a 1:1 fashion to Supraflex Cruz versus SYNERGY stents. Inclusion and exclusion criteria are listed in Supplementary Table 3.
STUDY ENDPOINTS
The primary endpoint for this trial is a non-inferiority comparison of the patient-oriented composite endpoint (POCE) of the Supraflex Cruz cohort to the SYNERGY cohort at 12 months post procedure. POCE14 is a composite clinical endpoint of all-cause death, any stroke, any MI, or any repeat revascularisation. The definition of MI will follow the Society for Cardiovascular Angiography and Interventions (SCAI) consensus for periprocedural MI ≤48 hours15, and Fourth Universal Definition (FUD) for MI >48 hours after the index procedure16. The powered secondary endpoint is a superiority comparison in the per-protocol analysis - at the vessel level - of the vessel-oriented composite endpoint (VOCE)17, a composite of vessel-related cardiovascular death, vessel-related MI, or clinically and physiologically indicated target vessel revascularisation (CPI-TVR), at 24 months post procedure. Other secondary endpoints are described in Supplementary Table 4,14,18.
QFR AND FUNCTIONAL SYNTAX SCORE ANALYSIS IN CORE LAB
QFR will be analysed off-line by QAngio XA 3D/QFR imaging software (Medis Medical Imaging Systems, Leiden, the Netherlands) before randomisation, and identification of functionally significant lesions will be provided to sites as an indication for treatment (Supplementary Figure 2). Details of the QFR analysis are described in Supplementary Appendix 4. At that time, the anatomical SYNTAX score I and functional SYNTAX score will be calculated by an independent core lab (CORRIB Core Lab, Galway, Ireland). The functional SYNTAX score will be used to generate the SYNTAX score III that predicts the major adverse cardiac and cerebrovascular events (MACCE) rate as well as the all-cause mortality in patients undergoing PCI5,7,19.
DEVICES
The Supraflex Cruz is the next-generation Supraflex stent. It is designed with open cells and dual valley-to-valley links between strut rings (Supplementary Figure 3A). The strut thickness is 60 μm across all diameters (2.00-4.5 mm). The conformal coating layer comprises the drug blended with a biodegradable polymeric matrix. The average thickness of the coating ranges from 4 to 6 μm. The Supraflex Cruz is coated with sirolimus at a concentration of 140 μg/cm2. The drug is 80% released within four weeks; the remainder is released over a period of three months. The polymers gradually degrade in 10 to 12 months. Supplementary Figure 3B displays the mechanical characteristics of the Supraflex Cruz, as documented according to the International Organization for Standardization (ISO), compared to other commercially available drug-eluting stents4.
The SYNERGY stent is used as the control device (Supplementary Figure 3A, Supplementary Figure 3B).
INDEX AND STAGED PROCEDURES
With respect to CTO revascularisation, a locally accredited expert will be selected in all centres to be part of the Multivessel TALENT team, and contemporary CTO techniques10 will be applied. In case of stent delivery failure, it is recommended first to try the comparator stent (crossover)18.
The use of IVUS/OCT pre PCI will be left to the discretion of the investigator; however, IVUS/OCT for optimising stent implantation after stent deployment is mandated9. Supplementary Figure 4 shows the criteria for stent optimisation.
Staged procedures are permitted and will be encouraged for more complex cases (e.g., revascularisation of total occlusions) to increase the likelihood of complete revascularisation and to decrease the risk of contrast-induced nephropathy20. The recommended timing of all planned elective staged PCI procedures is within two weeks post index procedure (with an upper limit of eight weeks). When the staged procedure is performed beyond eight weeks, such a procedure is considered as a clinical event. Staged procedures are only allowed in non-target vessels. The patient should receive the stents assigned during the original index procedure.
DEVICE AND PROCEDURE SUCCESS
Device success (lesion basis) is defined as successful delivery and implantation of the assigned device in the intended location with the final residual stenosis being less than 20% (preferably by quantitative coronary angiography [QCA]). Procedure success is defined as device success without POCE or stent thrombosis during the index procedure hospital stay (maximum of seven days)18.
ADJUNCTIVE PHARMACOLOGICAL THERAPY
Preloading with aspirin 300 to 325 mg at least two hours before the PCI is mandatory unless the patient already receives chronic aspirin therapy. Prasugrel preloading therapy is also mandatory. For patients already receiving chronic prasugrel therapy, preloading with a dose of 60 mg of prasugrel is mandatory at least two hours before the PCI procedure. Switching from clopidogrel or ticagrelor to prasugrel should be conducted according to the consensus document21 (Supplementary Figure 5). In addition, atorvastatin 80 mg, rosuvastatin 40 mg, or a PCSK-9 inhibitor must be administered at least 24 hours before the PCI, regardless of low-density lipoprotein (LDL) level, if not taking any statin at a maximum dose in the 24 hours prior to the loading dose22.
ANTIPLATELET THERAPY AFTER PCI
After PCI, all patients must receive DAPT with aspirin and prasugrel for one month, followed by 11 months of prasugrel monotherapy. At one year, prasugrel monotherapy should be replaced by aspirin monotherapy (Supplementary Figure 1). The dose of aspirin and prasugrel will be 75-100 mg and 10 mg per day, respectively. The dose of prasugrel should be decreased to 5 mg in patients with a weight <60 kg or age >75 years23.
STATISTICAL ANALYSIS
For the primary analysis of the primary endpoint, the intention-to-treat population will be used: all patients will be analysed according to their assigned treatment group, regardless of the treatment actually received. The proportion of patients reaching a POCE at 12 months in each study arm will be estimated using a Kaplan-Meier estimator. A one-sided 95% confidence interval of the difference in weighted proportions will be compared to the non-inferiority limit (absolute risk increase of 4.28%), with a corresponding one-sided p-value for non-inferiority to be reported.
The primary analysis of the powered secondary endpoint will be a per-protocol analysis at vessel level. The definition of the per-protocol population is shown in Supplementary Appendix 5. The proportion of vessels reaching a VOCE by 24 months in each study arm will be estimated using a Kaplan-Meier estimator. Cluster-robust standard errors will be used to account for the correlation of VOCE measurements within a patient. A two-sided, cluster-robust 95% confidence interval of the difference in proportions will be compared to zero difference, with a corresponding two-sided p-value for superiority to be reported.
Other secondary endpoints will be analysed in the intention-to-treat principle as appropriate (according to the assumed distribution of each outcome). For these analyses, the focus will be on the point estimates and confidence intervals for hypothesis generation.
A secondary analysis of the primary endpoint and all its secondary clinical endpoints will also be conducted in the as-treated and per-protocol population. The definition of the as-treated population is shown in .
SAMPLE SIZE CALCULATION
Assuming a 1:1 treatment allocation ratio, a one-sided significance level (alpha) of 0.05, a POCE event rate for SYNERGY of 10.7% at 12 months in 3VD5, a non-inferiority margin of 4.28% (risk ratio: 1.4), and no difference in event rate between the two groups, 751 patients per arm are required to achieve 85% power to show non-inferiority of the Supraflex Cruz to the SYNERGY. Taking into account an attrition rate of approximately 3%, these numbers increase to 775 in each group, giving a total randomised sample of 1,550 patients.
The powered secondary analysis will be conducted to test superiority in VOCE for the Supraflex Cruz, compared to SYNERGY, at the vessel level on a per-protocol principle17. Based on analysis from the SYNTAX II trial5, the proportion of per-protocol vessels with a VOCE at two years was assumed to be 6.51% in the SYNERGY arm, and the correlation of multiple VOCE measurements within patients was 0.45. A minimally important effect of a relative reduction by 37.5% in the Supraflex Cruz arm (VOCE: 4.07%) was chosen. Given that the sample size is fixed for the primary outcome at 1,550 participants and assuming an attrition rate of 5% before two years, this gives 1,472 participants with VOCE measurements for analysis. Within-patient correlation of VOCE results in a design effect of 1.54 (assuming a mean of 2.2 vessels per patient which are treated with PCI)24. Thus, the effective sample size of all vessels in analysis is 1,472*2.2/1.54=2,103. A test of statistical superiority for a difference in proportions uses cluster-robust standard errors with a type-1 error of 0.05 and these parameters will thus have a power of 80%.
PRE-SPECIFIED SUBGROUP ANALYSES
Pre-specified subgroup analyses are listed in Supplementary Appendix 6. For these analyses, the study does not have significant power to demonstrate non-inferiority/superiority for the Supraflex Cruz arm over the SYNERGY arm, meaning that the results are considered exploratory (hypothesis-generating) only.
Discussion
The Multivessel TALENT trial compares clinical outcomes between novel ultra-thin Supraflex Cruz and SYNERGY stents in patients with de novo 3VD without left main disease, applying the five treatment principles of “best practice” PCI6. Our hypothesis is based on the results of the per-protocol analysis of the TALENT trial that indicates a 61% reduction in clinically indicated TLR1. In addition, a meta-analysis also demonstrated that newer-generation ultra-thin strut DES significantly reduced target lesion failure driven by fewer procedural and spontaneous myocardial infarctions, compared with thicker-strut second-generation DES25.
Treatment strategies for complex coronary artery disease have been improved since the enrolment period of the SYNTAX II study13,26. First, patient selection is based on SYNTAX score II recommendations7 and Heart Team consensus5,27. Second, novel physiological methods to assess ischaemia have been developed12. The diagnostic accuracy of QFR, that does not require pharmacologic hyperaemia induction, for identifying an FFR of ≤0.80 has been demonstrated in the FAVOR Pilot study. Thereafter, the FAVOR II China and FAVOR II Europe-Japan studies also demonstrated the diagnostic accuracy of QFR for detecting functionally significant lesions in comparison with 2D-QCA, using FFR as reference standard28. In a systematic review and Bayesian meta-analysis, Collet et al confirmed the high sensitivity and specificity of QFR against pressure wire-derived physiological assessment12. Therefore, the systematic physiological assessment for all vessels by QFR becomes reasonable in terms of cost, time, and safety. Third, the benefit of IVUS-guided PCI has been demonstrated in the ULTIMATE and IVUS-XPL trials29, and intravascular imaging for post-stent optimisation is recommended in an expert consensus document9. Fourth, the presence of a CTO was the strongest independent predictor of incomplete revascularisation in the PCI arm of the SYNTAX trial30. Operator skill and use of specific techniques and devices are key determinants of PCI success5; therefore, a locally accredited expert in CTO is recommended to be selected in all participating centres, and an algorithm of treatment is advised31. Regarding antiplatelet therapy after PCI as a part of optimal medical treatment, the sub-analysis of the GLOBAL LEADERS trial and the TWILIGHT study demonstrated the clinical benefit of ticagrelor monotherapy after short DAPT in patients with 3VD13,26. The ISAR-REACT 5 trial demonstrated that, in patients who presented with acute coronary syndromes, the incidence of death, MI, or stroke was significantly lower among those who received prasugrel than among those who received ticagrelor23. Since the landmark analysis at one year did not demonstrate any difference between ticagrelor monotherapy and aspirin monotherapy in clinical outcomes during the second year of the GLOBAL LEADERS trial, the monotherapy with prasugrel will be interrupted after one year and switched back to aspirin monotherapy. Therefore, in this 3VD trial, the antiplatelet therapy will be as follows: DAPT with aspirin and prasugrel for one month, followed by 11 months of prasugrel monotherapy, replaced by aspirin monotherapy at one year.
Limitations
Patients with de novo 3VD will be treated according to state-of-the-art PCI. As previously stated for the SYNTAX II trial, it will not be possible to identify the most influential factor of clinical benefit among the five treatment principles of best practice. On the other hand, this is a prospective, randomised trial comparing clinical outcomes between Supraflex Cruz and SYNERGY stents and the non-inferiority or even the superiority of one device versus the other will be the most tangible result of this trial.
There is no consensus on the width of a non-inferiority margin in non-inferiority trials32. In the TALENT and DESSOLVE III trials, both non-inferiority margins were 4.0% (risk ratio: 1.5) in the DOCE (cardiac death, target vessel MI, and clinically indicated TLR)1,33. A non-inferiority margin of 4.28% (relative risk ratio: 1.4) for POCE at 12 months is more stringent than in the above-mentioned trials.
Conclusions
The Multivessel TALENT trial will assess state-of-the-art PCI for complex coronary artery disease. It will develop “best practice” PCI in terms of physiological assessment using QFR, apply a novel antiplatelet therapy strategy, and subsequently assess the non-inferiority and possibly the superiority of the novel ultra-thin Supraflex Cruz stent compared to the SYNERGY stent.
|
Impact on daily practice This study could establish PCI with the novel ultra-thin Supraflex Cruz stent as an attractive option for revascularisation in patients with de novo 3VD without left main disease. |
Funding
The Multivessel TALENT trial is an investigator-initiated trial sponsored by The National University of Ireland Galway which received funding from SMT (Sahajanand Medical Technologies, Mumbai, India). The role of the funding source and the responsibilities of the sponsor are outlined in Supplementary Appendix 7.
Conflict of interest statement
H. Hara reports a grant for studying overseas from the Japanese Circulation Society, a grant-in-aid for JSPS Fellows and a grant from the Fukuda Foundation for Medical Technology. J.H.C. Reiber is Chief Scientific Officer of Medis Medical Imaging Systems bv, the company that developed the QFR. A. Zaman reports consultancy and/or lecture fees from SMT, Boston Scientific, Abbott Vascular, and HeartFlow. W. Wijns reports institutional grants from SMT and MicroPort, and personal fees from MicroPort, being a co-founder of Argonauts, an innovation facilitator, and being a medical advisor to Rede Optimus Research. P.W. Serruys reports personal fees from Biosensors, Micel Technologies, Sino Medical Sciences Technology, Philips/Volcano, Xeltis, and HeartFlow, outside the submitted work. M. Sabaté has received consultancy fees from Abbott Vascular and iVascular outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

