Abstract
Background: In the TALENT study, the sirolimus-eluting ultrathin strut Supraflex stent was non-inferior to the XIENCE stent for a device-oriented composite endpoint (DoCE: defined as cardiac death, target vessel myocardial infarction [TV-MI], or clinically indicated target lesion revascularisation [CI-TLR]) at 12 months.
Aims: This study investigated the 3-year outcomes of the TALENT trial and long-term impact of ultrathin drug-eluting stents (DES), compared to the XIENCE everolimus-eluting thin stent.
Methods: The TALENT trial is a prospective, multicentre, randomised all-comers trial comparing the Supraflex sirolimus-eluting stent with the XIENCE everolimus-eluting stent, with planned follow-up for 3 years.
Results: The TALENT trial enrolled 1,435 patients (Supraflex n=720, XIENCE n=715) with 3-year follow-up data available in 97.8% in the Supraflex group, and in 98.9% in the XIENCE group. At 3 years, DoCE occurred in 57 patients (8.1%) in the Supraflex group, and in 66 patients (9.4%) in the XIENCE group (p=0.406). There were no significant between-group differences in rates of cardiac death, TV-MI or CI-TLR. The rates of definite or probable stent thrombosis were low and similar between groups (1.1% vs 1.4%; p=0.640). In a meta-analysis of long-term follow-up (3-5 years), ultrathin strut DES tended to reduce DoCE (relative risk 0.89 [0.79-1.01]; p=0.068), compared to thicker strut DES. The risks for cardiac death and definite or probable stent thrombosis were similar between ultrathin strut DES and thicker strut DES.
Conclusions: At 3-year follow-up, the use of the Supraflex stent was at least as safe and efficacious as the XIENCE stent in an all-comers population. ClinicalTrials.gov: NCT02870140
Introduction
Stents with thinner struts have been shown to reduce acute thrombogenicity and promote faster endothelialisation, compared to stents with thicker struts123. One hypothesis behind this is that protruding thicker struts disrupt laminar flow, inducing flow disturbances, which can activate a platelet-signalling procoagulation pathway14. The physiological benefits and improved fluid dynamics with thinner struts may be partly responsible for the reduced rates of restenosis, stent thrombosis, and myocardial infarction (MI) observed with contemporary second-generation drug eluting stents (DES), which all have strut thicknesses of <100 μm, when compared to first-generation DES, which had strut thicknesses of >132 μm. The development of ultrathin strut stents, with strut thicknesses of <70 μm may further improve event-free survival compared to thin strut DES (second-generation DES).
The Supraflex stent (Sahajanand Medical Technologies) is a sirolimus-eluting stent (SES) with a biodegradable polymeric coating and 60 μm ultrathin struts. In the TALENT study, the Supraflex SES was non-inferior to the XIENCE durable polymer everolimus-eluting stent (EES; Abbot Vascular), for a device-oriented composite endpoint (DoCE) of cardiac death, target vessel myocardial infarction (TV-MI), or clinically indicated target lesion revascularisation (CI-TLR) at 12 months56. The longer-term outcomes with ultrathin DES are currently limited, and therefore we investigated the final 3-year outcomes after implantation of the Supraflex SES as compared to the XIENCE EES in the TALENT all-comers trial.
Methods
Study design and population
The design and 2-year results of the TALENT trial have been reported previously567. In brief, the TALENT trial is a prospective, multicentre, single-blinded, all-comers, randomised controlled trial, allocating patients in a 1:1 ratio to either the Supraflex SES or XIENCE EES. Twenty-three sites in Europe enrolled patients from October 2016 to July 2017. The primary endpoint of the study was a non-inferiority comparison at 12 months of a DoCE, defined as a composite of cardiac death, TV-MI, and CI-TLR. The composite secondary endpoints were a patient-oriented composite endpoint (PoCE) of all-cause death, any MI, and any revascularisation, and target vessel failure (TVF), a composite of cardiac death, TV-MI, and clinically indicated target vessel revascularisation (CI-TVR). Stent thrombosis – a safety indicator – was defined as per the Academic Research Consortium definition8. MI was defined according to the Society for Cardiovascular Angiography and Interventions consensus for periprocedural MI (when occurring 48 hrs or less after the index procedure) or according to the Third Universal Definition for MI910. Clinical data were adjudicated by an independent clinical event committee, blinded to stent allocation.
Patients with stable coronary artery disease received dual antiplatelet therapy (DAPT) for >6 months after percutaneous coronary intervention (PCI), followed by aspirin monotherapy indefinitely. Patients with acute coronary syndrome received DAPT for >12 months after PCI, followed by aspirin monotherapy indefinitely. The protocol prespecified patient follow-up up to 3 years.
All patients provided written informed consent to participate in the study. The study protocol of the TALENT trial was approved by institutional ethics committees of participating institutions and central regulatory bodies for each country, and was conducted according to the Declaration of Helsinki and Good Clinical Practice.
Study stents
Supraflex is a new-generation metallic stent consisting of an L605 cobalt-chromium alloy platform with ultrathin struts (60 μm) across all stent diameters, flexible S-link connectors, and a biodegradable polymeric matrix coating. Sirolimus, at a concentration of 1.4 μg/mm², together with the polymeric matrix, is coated on the conformal surface of the stent, with an average coating thickness of 4-5 μm. Seventy percent of the sirolimus is eluted in the first 7 days, with the remainder released over the following 48 days. The polymer gradually degrades over 9-12 months. The crossing profile of the Supraflex is 0.99 mm (the crossing profile of the newest XIENCE Alpine EES is 1.10 mm and of the XIENCE Sierra EES is 0.99 mm).
The control stent used in the study was the XIENCE EES, which has a cobalt chromium alloy platform and a strut thickness of 81 μm. It has an 8 μm thick durable polymer coated with everolimus at a dose of 1 μg/mm², which is completed eluted over 120 days.
Meta-analysis
Randomised clinical trials comparing ultrathin strut DES (strut thickness <70 μm) and thicker strut DES (strut thickness ≥81 μm) with at least 3-year outcomes were searched on PubMed, EMBASE, and abstracts and presentations from major cardiovascular meetings between January 2010 and October 2021 (Supplementary Table 1). The meta-analytic summary estimates (relative risk [RR] with 95% confidence interval [CI]) for the ultrathin strut DES versus thicker strut DES in terms of DoCE, its individual components, definite or probable stent thrombosis, and all-cause death at the time of last available follow-up were evaluated using results reported in intention-to-treat (ITT) analyses. All outcomes were calculated using both the fixed-effects model and the random-effects model of DerSimonian and Laird11. This was done to compare the fixed- and random-effects estimates of the intervention as recommended by the Cochrane Collaboration, given that we anticipated some heterogeneity (I2 >0). If the estimates are similar, then any small-study effects have little impact on the intervention effect estimate. Heterogeneity was assessed using the I2 statistic, with I2 <25% considered low, I2 ≥25% and ≤75% considered moderate, and I2 >75% considered high1213. When heterogeneity was moderate or high, the L'Abbé plot was demonstrated. Publication bias was visually inspected using a funnel plot. Risk of bias was assessed according to the Cochrane Collaboration’s tool14.
Statistical analysis
All patients in the ITT analysis were analysed according to their assigned treatment group, regardless of the actual treatment received. Patients who were randomised to a treatment group and only received that assigned study stent were included in the per protocol (PP) analysis.
Prespecified subgroup analyses were performed for the primary endpoint, DoCE, with respect to diabetes, ST-segment elevation MI (STEMI), small vessels (≤2.75 mm), multivessel treatment, long lesions (>18 mm), in-stent restenosis, bypass graft, left main treatment, bifurcation treatment, or overlapping stents.
The cumulative event rates were estimated using the Kaplan-Meier method and comparisons of outcomes were performed with the log-rank test. Hazard ratios were calculated using the Cox proportional hazards model. P-values are for the superiority and a two-sided p-value <0.05 was considered statistically significant. Analyses were performed using SAS software, version 9.3 (SAS Institute Inc.) and R version 3.6.0 (R Foundation for Statistical Computing).
Results
Study population
The TALENT trial enrolled 1,435 patients with 2,076 lesions; 720 patients with 1,046 lesions were randomly assigned to Supraflex, and 715 patients with 1,030 lesions to XIENCE (Figure 1). Baseline clinical, angiographic, and procedural characteristics were comparable between the two groups, as previously reported15. Three-year follow-up data were available for 97.8% (704/720) of patients in the Supraflex group and for 98.9% (707/715) of patients in the XIENCE group (Figure 1).
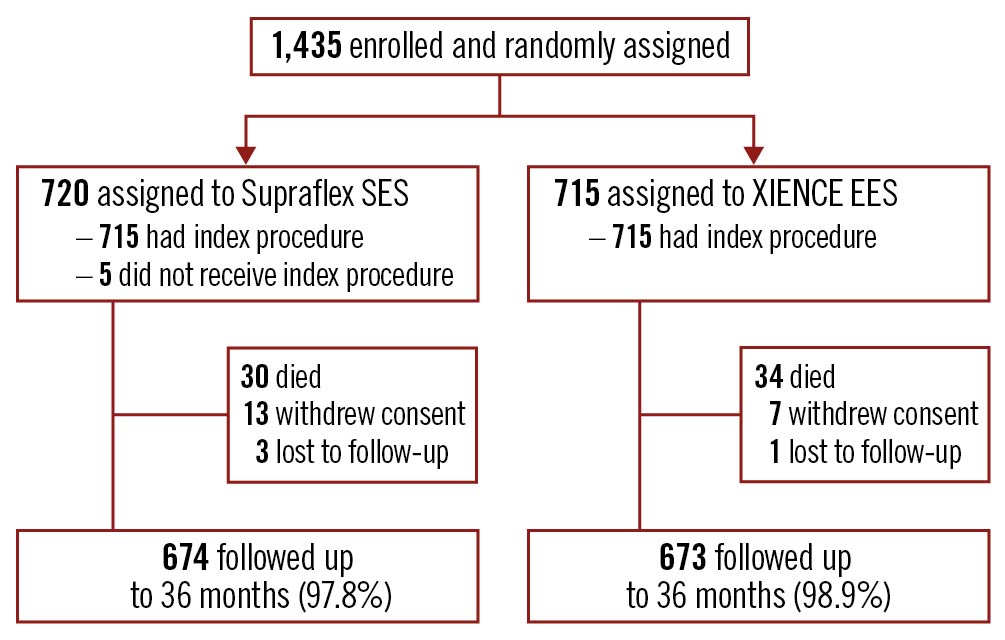
Figure 1. Study flowchart. EES: everolimus-eluting stent; SES: sirolimus-eluting stent.
Clinical outcomes at 3 years (ITT analysis)
At 3 years DoCE occurred in 57 patients (8.1%) in the Supraflex group, and in 66 patients (9.4%) in the XIENCE group (difference −1.3% [95% CI: −4.3% to 1.6%]; p=0.406) (Table 1, Figure 2A). There were no significant between-group differences in rates of cardiac death, TV-MI, and CI-TLR (Table 1, Figure 2B-Figure 2D). There were also no significant differences in the groups between 1 and 3 years (Supplementary Figure 1). The percentage of patients with DAPT at 6 and 12 months was similar (Supplementary Table 2), and the rates of definite or probable stent thrombosis were low and comparable (Supraflex 1.1% vs XIENCE 1.4%, difference −0.4% [95% CI: −1.5% to 0.7%]; p=0.640) (Table 1, Figure 2E). The rates of other clinical events are presented in Table 1. Non-TV revascularisation was significantly lower in the XIENCE group (5.7%), compared to the Supraflex group (8.6%) (difference 2.9% [95% CI: 0.2% to 5.6%]; p=0.035), although these events were not associated with lesions treated with study stents.
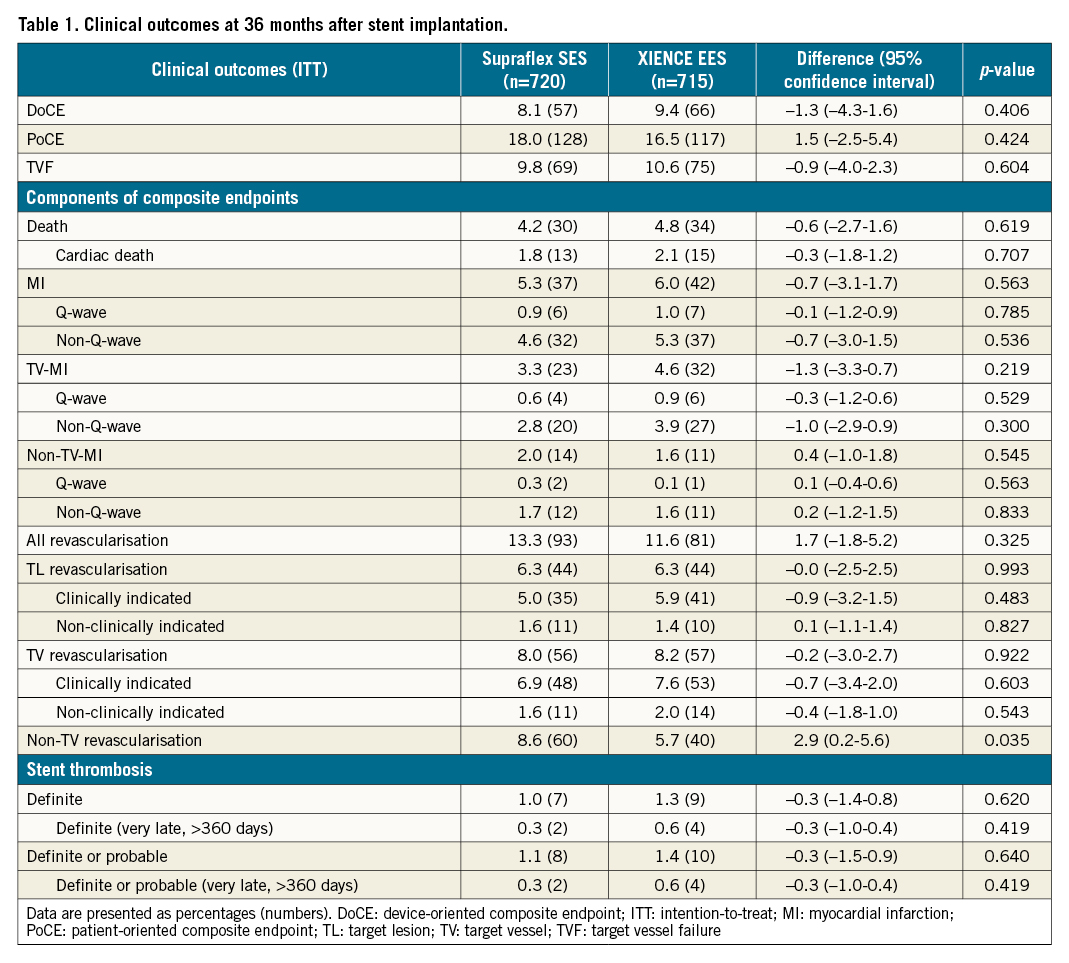
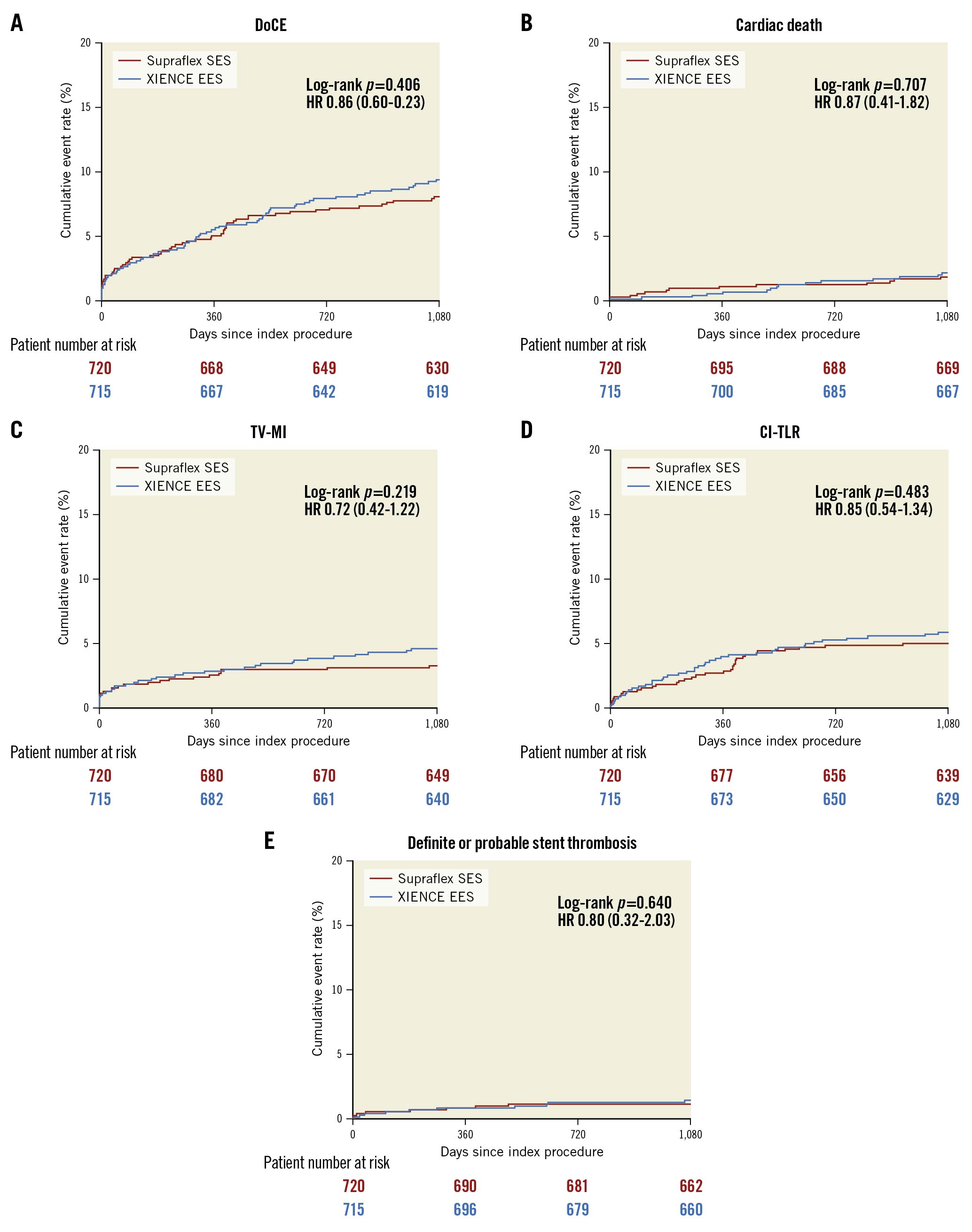
Figure 2. Kaplan-Meier estimates for the device-oriented composite endpoint (DoCE) and its components at 3 years (intention-to-treat [ITT] basis). A) DoCE, B) cardiac death, C) target vessel myocardial infarction (TV-MI), D) clinically indicated target lesion revascularisation (CI-TLR), and E) definite or probable stent thrombosis. HR: hazard ratio.
Per protocol (PP) analysis
In the PP analysis at 3 years DoCE occurred in 43 (6.6%) patients treated with Supraflex and 59 (8.7%) patients treated with XIENCE (difference −2.1% [95% CI: −5.0% to 0.8%], p=0.165) (Supplementary Table 3, Supplementary Figure 2A). The rates of cardiac death, TV-MI and CI-TLR were all numerically lower, but not statistically different with Supraflex compared with XIENCE. Notably the significantly lower rate of CI-TLR observed with Supraflex in the PP analysis at 1-year (1.2% vs 3.1%, difference −1.9% [95% CI: −3.5% to 0.3%], p=0.021)6 was no longer evident at 3 years (3.6% vs 5.1%, difference −1.5%, [95%CI: −3.7 to 0.7], p=0.192) (Supplementary Table 3, Supplementary Figure 2B-Supplementary Figure 2D). There were no significant differences between stents in rates of non-TV revascularisation (Supraflex 7.8% vs XIENCE 5.8%, difference 2.0% [95% CI: −0.7% to 4.7%], p=0.143).
Subgroup analysis
The treatment effect in DoCE was no different across the prespecified subgroup analyses for diabetes, STEMI, multivessel treatment, long lesions, in-stent restenosis, bypass graft, left main treatment, bifurcation treatment, or overlapping stents, although Supraflex resulted in better outcomes in patients without small vessels treated (Figure 3).
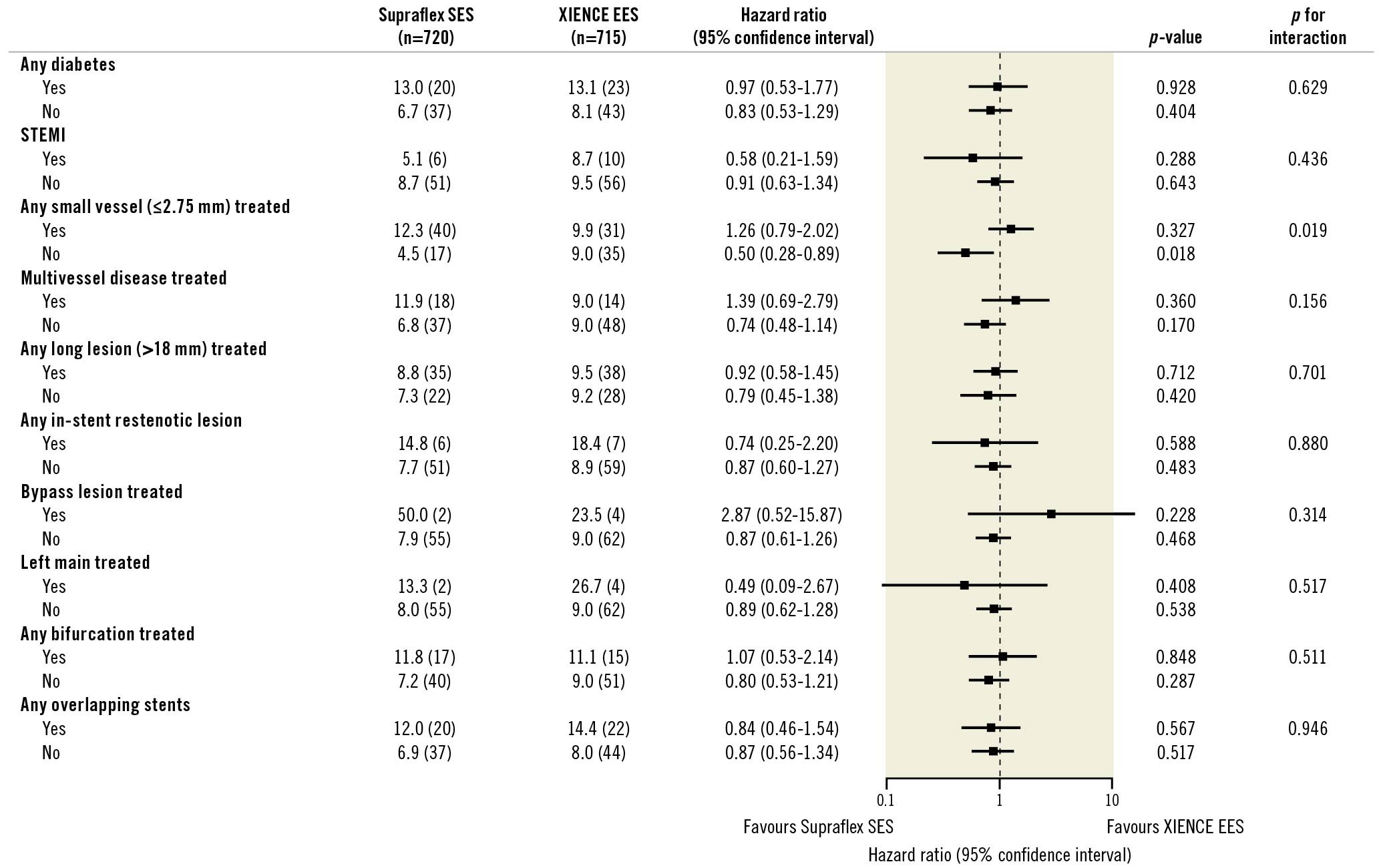
Figure 3. Subgroup analysis for DoCE (ITT basis). DoCE: device-oriented composite endpoint; ITT: intention-to-treat; STEMI: ST-elevation myocardial infarction
Meta-analysis
Including the TALENT trial, there were 11 randomised trials (15,370 patients) with at least 3-year results comparing outcomes between ultrathin strut DES with thicker strut DES (Table 2, Supplementary Table 4, Supplementary Figure 3). Overall, ultrathin strut DES resulted in an 11% reduction in DoCE compared to thicker strut DES (RR 0.89, 95% CI: 0.79-1.01; p=0.068), although the effect was not statistically significant (Figure 4). Ultrathin strut DES and thicker strut DES had similar risks for definite or probable stent thrombosis and mortality (Figure 4). Moderate heterogeneity was observed for DoCE and death; thus the L'Abbé plots are presented in Supplementary Figure 4. The funnel plots and risk of bias are shown in Supplementary Figure 5 and Supplementary Table 4.
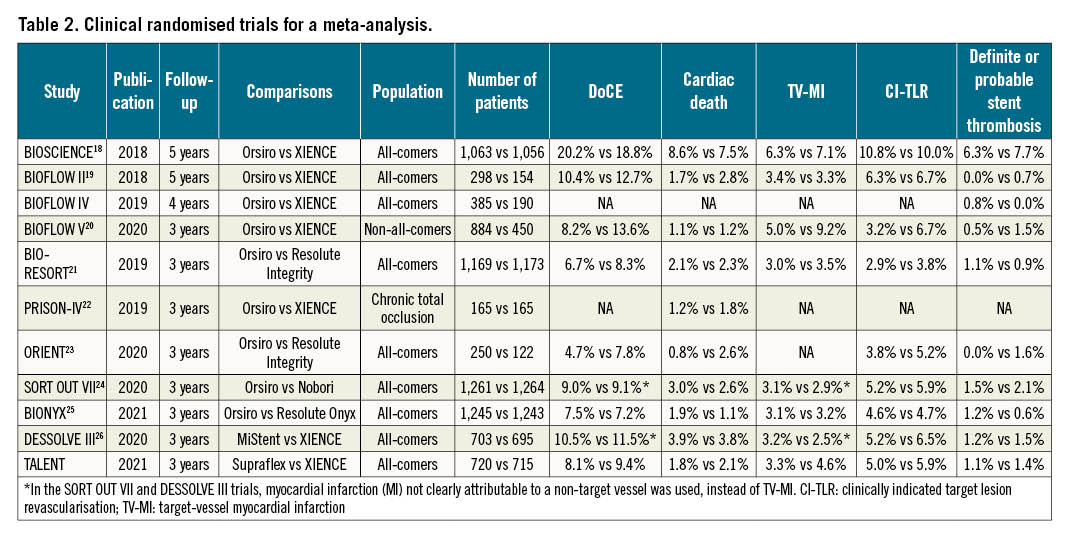
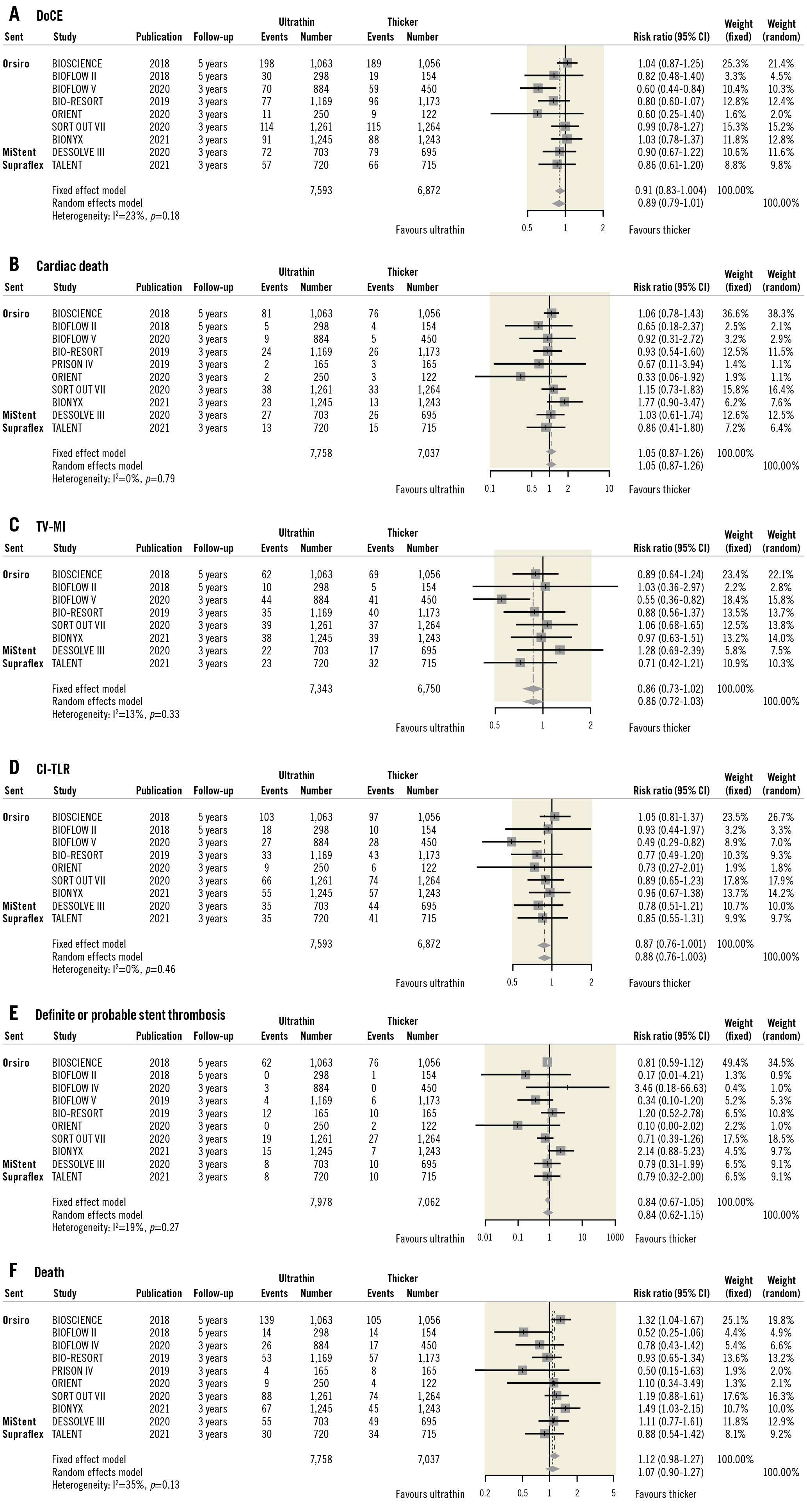
Figure 4. Long-term outcomes of ultrathin strut DES vs thicker strut DES. A) DoCE, B) cardiac death, C) TV-MI, D) CI-TLR, E) definite or probable stent thrombosis, and F) death. In the BIOFLOW V trial, DoCE was defined as cardiovascular death, TV-MI, or ischaemia-driven TLR. In the SORT OUT VII and DESSOLVE III trials, MI not clearly attributable to a non-target vessel was used instead of TV-MI. CI: confidence interval; CI-TLR: clinically indicated target lesion revascularisation; DoCE: device-oriented composite endpoint; TV-MI: target vessel myocardial infarction
In patients with diabetes or small vessels treated, there were no statistically significant differences in DoCE between ultrathin strut DES and the thicker strut DES (Supplementary Figure 6).
Discussion
At 3-year follow-up of the randomised all-comers TALENT trial, there were no significant differences in rates of DoCE, its individual components, or stent thrombosis between patients assigned to the Supraflex or XIENCE groups (Central illustration, panel A).
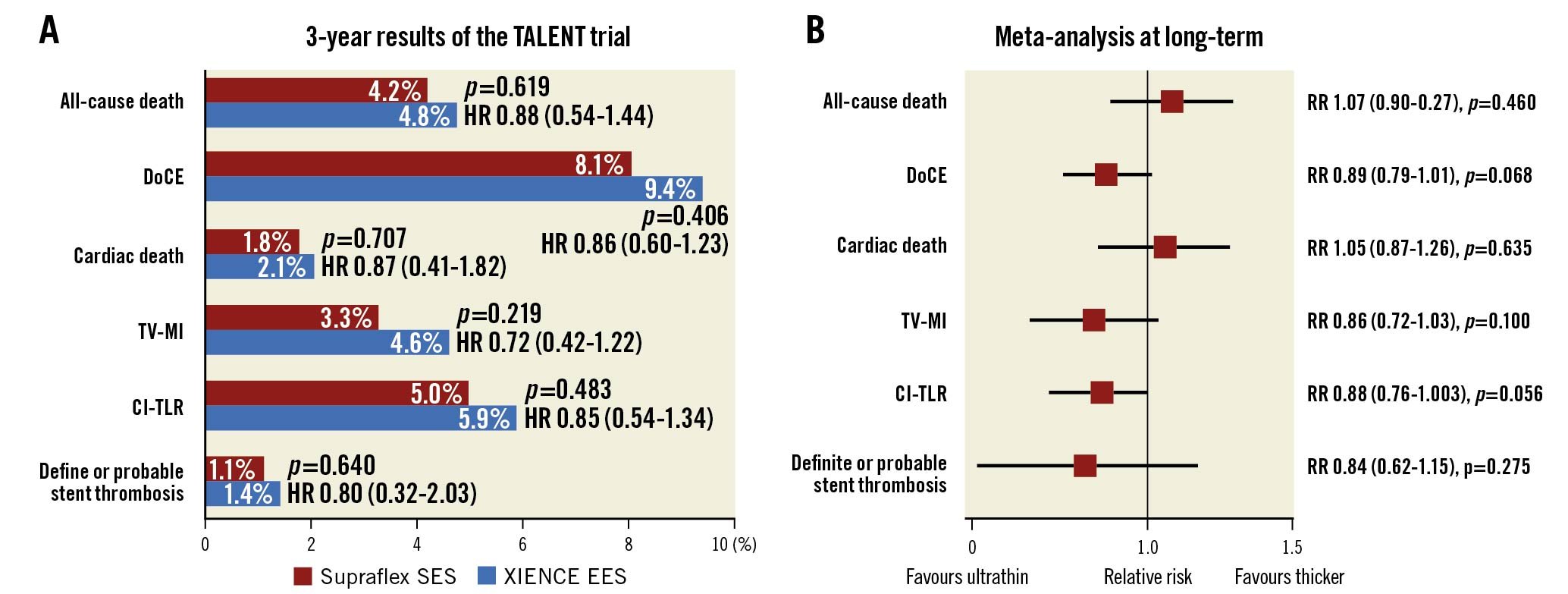
Central illustration. Results of the TALENT trial and a long-term meta-analysis. A) Three-year results of the TALENT trial. B) Long-term (3-5 years) results of a meta-analysis. CI: confidence interval; CI-TLR: clinically indicated target lesion revascularisation; EES: everolimus-eluting stent; DoCE: device-oriented composite endpoint; HR: hazard ratio; RR: relative risk; SES: sirolimus-eluting stent; TV-MI: target vessel myocardial infarction
Impact of the Supraflex stent on repeat revascularisation
At 1-year follow-up in the PP analysis, the Supraflex stent resulted in a significantly lower rate of CI-TLR, compared to XIENCE. At 3-year follow-up, whilst the rate of CI-TLR was still numerically lower with Supraflex, the difference was no longer statistically significant (5.0% vs 5.9%; p=0.483 [ITT analysis]; 3.6% vs 5.1%; p=0.192 [PP analysis]). Longer follow-up and/or a larger sample size are certainly needed to fully examine how this early difference could be more durable.
Impact of ultrathin strut polymers
A meta-analysis of 10 randomised trials including 11,658 patients by Bangalore et al demonstrated that at 1-year ultrathin strut DES (Orsiro, MiStent, and BioMime) resulted in a 16% RR reduction in DoCE (RR 0.84, 95% CI: 0.72-0.99), compared to second-generation DES with thicker struts (XIENCE, Resolute, and Nobori)16. Recently, another meta-analysis at a mean follow-up of 2.5 years demonstrated that ultrathin strut DES reduced the risk of DoCE (RR 0.85, 95% CI: 0.76-0.96), driven by less CI-TLR (RR 0.75, 95% CI: 0.62-0.92) compared with second-generation DES with thicker struts, with similar risks of cardiac death and all-cause death17.
In the TALENT trial, the ultrathin strut Supraflex stent reduced DoCE at 1 year by 6%, compared to the thin strut XIENCE stent in the ITT analysis6. The effect of the ultrathin strut Supraflex stent was retained at 3 years with 14% risk reductions in DoCE, although the effect was not statistically significant.
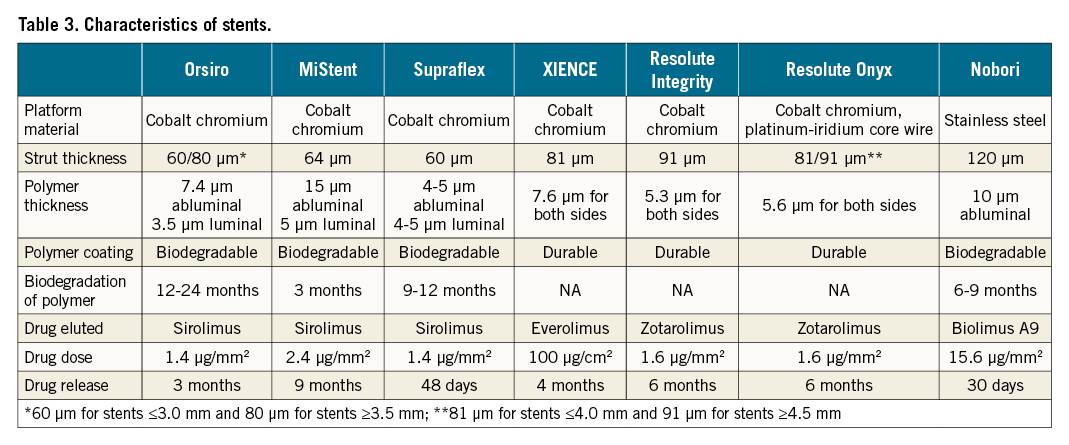
To date, long-term follow-up data with at least 3-year results of ultrathin strut stents (strut thickness <70 μm) versus thicker strut stents (strut thickness ≥81 μm) are available in the BIOSCIENCE18, BIOFLOW II19, BIOFLOW V20, BIO-RESORT21, PRISON-IV22, ORIENT23, SORT OUT VII24, BIONYX25 (Orsiro), DESOLVE III26 (MiStent), and TALENT (Supraflex) randomised trials. The 4-year results of BIOFLOW-IV have not been published, but have been presented at TCT by Slagboom et al. [TCT-43 A Prospective Randomized Multicenter Study to Assess the Safety and Effectiveness of the Orsiro Sirolimus-Eluting Stent in the Treatment of Subjects With Up to 2 De Novo Coronary Artery Lesions –BIOFLOW IV: 4-Year Clinical Results. J Am Coll Cardiol. 2019;74:B43]. The characteristics of these ultrathin strut stents are shown in Table 3162728.
Our updated meta-analysis of these trials, including results from the current study, demonstrates the safety of ultrathin strut DES compared to thicker strut DES at a minimum of 3 years of follow-up (Central illustration, panel B). Although moderate heterogeneity was observed between studies and the difference was not statistically significant, ultrathin strut DES reduced DocE by 11%, compared to thicker strut DES (RR 0.89, 95% CI: 0.79-1.01; p=0.068). The risks for cardiac death and definite or probable stent thrombosis were similar between ultrathin strut DES and thicker strut DES. Theoretically, thinner struts could have some advantages, such as: less stent-induced vessel injury and subsequent inflammation; faster re-endothelialisation; and less flow disturbance and fewer areas of low shear stress behind struts, resulting in reduced thrombogenicity123429. The stent strut thickness of Orsiro is 80 μm for stent diameters ≥3.5 mm, which is similar to the stent strut thickness of XIENCE (81 μm for all sizes) and Resolute Onyx (81 μm for stent diameters ≤4.0 mm). The patients treated with Orsiro with a stent diameter ≥3.5 mm may dilute the impact of stent strut thickness. In the BIOSCIENCE trial, 244 patients (23.0%) were treated with stents ≥3.5 mm in the Orsiro group. Thus, the meta-analysis may underestimate the impact of stent strut thickness, and the analysis using individual patient data is mandatory to investigate the impact of ultrathin strut DES precisely.
Comparison between newer-generation ultrathin strut DES
There are notable differences in stent profiles amongst the ultrathin strut Orsiro, MiStent, and Supraflex DES. The Supraflex and MiStent DES have a fixed strut thickness of 60 and 64 μm, respectively, irrespective of the stent diameter, which is at variance with the Orsiro stent, which has a strut thickness of 60 μm for stents 2.25 to 3.0 mm in diameter and 80 μm for stents with a diameter of 3.5 to 4.0 mm. Moreover, whilst these ultrathin strut stents all have biodegradable polymers and elute sirolimus, there are fundamental differences in their drug release kinetics. In the Supraflex stent, 70% of the sirolimus is eluted in the first 7 days during an initial burst, followed by sustained release which is completed by day 48; the polymer gradually degrades over 9-12 months. In the MiStent, no drug release occurs in the first 3 days, and whilst the polymer is fully biodegraded and resorbed within 3 months of implantation, microcrystalline sirolimus is impacted and embedded in the vessel wall, acting as a tissue reservoir for 270 days, such that arterial concentrations of sirolimus still reach more than 2 ng/ml at day 270. In the Orsiro stent, sirolimus is slowly released over 12-14 weeks, whilst its polymer completely degrades within 12-24 months. Although the rate of DoCE at 3 years with the MiStent in the all-comers DESSOLVE III trial was 10.2% (72 patients out of 703 patients, Kaplan-Meier estimated rate 10.5%), the rate of DoCE at 3 years was lower in the all-comers population treated with the Supraflex stent (57 patients [7.9%; Kaplan-Meier estimated rate 8.1%] out of 720 patients in the TALENT trial) (Table 2). The rate of DoCE at 3 years in the all-comers population treated with Orsiro was available in the BIO-RESORT, ORIENT, SORT OUT VII, and BIONYX trials, and was 7.5% (293 patients out of 3,925 patients).
Limitations
The TALENT trial was single-blinded, although the effect of this approach on event reporting is minimal because of the adjudication by an independent blinded clinical event committee. The study did not have adequate statistical power for any individual endpoints due to its relatively small sample size.
In terms of meta-analysis, the definition of DoCE was not the same in each trial (e.g., TV-MI or MI not clearly attributable to a non-target vessel, etc). The definition of MI was not consistent across trials (e.g., SCAI definition, universal definition of myocardial infarction, WHO’s extended definition, criteria of cardiac biomarkers, etc). Furthermore, long-term results of DoCE were not available for the BIOFLOW-IV and PRISON IV trials. Longer-term follow-up and large-scale individual data are necessary to investigate long-term benefits of ultrathin strut DES.
Conclusions
In the present final report of the TALENT trial, the use of the Supraflex ultrathin strut stent was at least as safe and efficacious as the XIENCE stent at 3 years in an all-comers population.
Impact on daily practice
The Supraflex ultrathin strut stent was at least as safe and efficacious as the XIENCE stent at 3 years in an all-comers population. In a meta-analysis of long-term follow-up (3-5 years), ultrathin strut DES were also as safe and efficacious as thicker strut DES. Ultrathin strut DES can be considered for PCI.
Funding
This study was sponsored by the European Cardiovascular Research Institute (ECRI, Rotterdam, the Netherlands), and supported with an unrestricted grant from Sahajanand Medical Technologies (Surat, India). ECRI funded the independent clinical research organisation Cardialysis for site management, safety reporting, data management, endpoint adjudication, database management, and statistical analyses.
Role of the Funder/Sponsor
The study funders had no role in trial design, data collection, analysis, interpretation of the data, preparation, approval, or making a decision to submit the manuscript or publication.
Acknowledgements
The authors would like to thank Anita van der Wal and Maurice Vorage as representatives of the study team at Cardialysis, Rotterdam for their operational contribution.
Conflict of interest statement
H. Hara reports a grant for studying overseas from Japanese Circulation Society, a grant-in-Aid for JSPS Fellows, a grant-in-aid from the Japan Foundation for Applied Enzymology and a grant from the Fukuda Foundation for Medical Technology. P. Smits reports grants and personal fees from Abbott Vascular, St. Jude Medical, and Terumo, outside the submitted work. S. Hofma reports unrestricted research grants from Abbott Vascular to The Research Department of the Division of Cardiology of the Medical Center Leeuwarden. R. Moreno reports lecture and consultant fees from Abbott Vascular, Boston Scientific, Biosensors, Biotronik, Daiichi Sankyo, Ferrer, Philips, and Edwards Lifesciences; and research grants from Abbott Vascular, Boston Scientific, Biosensors, Daiichi Sankyo, all outside of the submitted work. A. Zaman reports lecture fees from SMT and lecture/consulting fees from Abbott. I. Petrov reports lecture fees and proctor honoraria from Medtronic, Contego, Edwards Lifesciences, Amgen, Cardiatis, Abbott Vascular and Novartis, outside the submitted work. P.W. Serruys reports institutional grants from Philips/Volcano, Xeltis, Meril Life, Novartis and SMT, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

