Abstract
Aims: We evaluated the association between atherosclerotic plaque characteristics (APCs) by CT –including positive remodelling (PR), low attenuation plaque (LAP) and spotty calcification (SC)– and lesion ischaemia by fractional flow reserve (FFR).
Methods and results: Two hundred and fifty-two patients (17 centres, five countries) underwent CT, FFR derived from CT (FFRCT) with invasive FFR performed for 407 coronary lesions. FFR ≤0.8 was indicative of lesion-specific ischaemia. CT diameter ≥50% stenosis was considered obstructive. APCs by CT were defined as: (1) PR, lesion diameter/reference diameter >1.10; (2) LAP, any voxel <30 HU; and (3) SC, nodular calcified plaque <3 mm. Odds ratios (OR) and area under the ROC curve (AUC) of APCs for lesion-specific ischaemia were analysed. PR, LAP and SC were associated with ischaemia, with a three to fivefold higher prevalence than in non-ischaemic lesions. Among individual APC, PR (OR 4.7, p<0.001), but not SC or LAP, was strongly associated with lesion-specific ischaemia and provided incremental prediction for lesion-specific ischaemia over CT stenosis plus FFRCT (AUC 0.87 vs. 0.83, p=0.002).
Conclusions: APCs’ features –especially PR– by CT improve identification and reclassification of coronary lesions which cause ischaemia over CT stenosis and FFRCT.
Introduction
Coronary computed tomography (CT) angiography has emerged as a non-invasive method for accurate detection and exclusion of high-grade coronary stenoses, as compared to the invasive coronary angiography (ICA) reference standard1. Although current-generation CT exhibits high negative predictive value to exclude obstructive coronary artery stenosis, CT has a limited role in the assessment of the physiologic significance of lesions: fewer than half cause ischaemia2. In addition to luminal diameter narrowing, CT also enables assessment of several coronary atherosclerotic plaque characteristics (APCs) with high accuracy including positive arterial remodelling (PR), low attenuation plaque (LAP) as a marker for necrotic lipid-laden intraplaque core, and spotty intraplaque calcification (SC)3. Similar to invasive studies by intravascular ultrasound (IVUS), these CT characteristics have been associated with culprit lesions in retrospective and prospective cohorts4,5. Recently, computation of fractional flow reserve (FFR) from CT (FFRCT) has emerged as a novel non-invasive method which demonstrates high diagnostic performance for identification and exclusion of patients and coronary lesions that cause ischaemia6-8. FFRCT demonstrated greater discrimination over CT stenosis alone for the diagnosis of lesions that cause ischaemia7,8.
FFR is a useful method for physiologic assessment of coronary artery lesions and represents the current gold standard for identification of lesions which cause ischaemia9,10. Prior FFR studies have demonstrated the importance of this physiologic measure, with FFR identification of high-risk ischaemia-causing lesions associated with a higher rate of cardiovascular events, and with FFR-guided coronary revascularisation enhancing event-free survival in a cost-effective manner11. The use of FFR has established stenosis severity as an unreliable indicator of ischaemia, with approximately half of all high-grade stenoses manifesting no ischaemia. Conversely, a significant proportion of lesions considered anatomically non-obstructive do, in fact, cause ischaemia by FFR12. These findings suggest the importance of other factors beyond luminal diameter narrowing as critical to the identification of lesion-specific ischaemia.
Whether APCs by CT are associated with specific coronary lesions which cause ischaemia over CT stenosis and FFRCT remains unknown. In a prospective international multicentre study, we thus examined the relationship of APCs by CT to lesion-specific ischaemia by FFR.
Methods
STUDY POPULATION
We studied 252 consecutive patients from the Determination of Fractional Flow Reserve by Anatomic Computed Tomographic AngiOgraphy (DeFACTO) study (NCT01233518), which was performed at 17 centres in five countries (Belgium [n=1], Canada [n=1], Latvia [n=1], South Korea [n=2], and the USA [n=12])7,13. The rationale and design of the DeFACTO study has been previously described7,13. Enrolled patients were adults with suspected coronary artery disease (CAD) who underwent clinically indicated ICA within 60 days after CT with no intervening coronary event.
ICA AND FFR MEASUREMENTS
ICA and FFR measurements have been previously described7,13. In brief, ICAs were evaluated by quantitative coronary angiographic (QCA) stenosis severity using a blinded angiographic core laboratory (University of British Columbia, Vancouver, Canada) using commercially available software (Discovery Quinton). FFR was performed at the time of ICA (PressureWire™ Certus™; St. Jude Medical, St. Paul, MN, USA; ComboWire®; Volcano Corp, Rancho Cordova, CA, USA). Investigators performed FFR in vessels deemed clinically indicated for evaluation and demonstrating an ICA stenosis between 30% and 90%. After administration of nitroglycerine, a pressure monitoring guidewire was advanced distal to the stenosis. Hyperaemia was induced by administration of intravenous adenosine at a rate of 140 µg/kg/min. FFR was calculated by dividing the mean distal coronary pressure by the mean aortic pressure during hyperaemia. FFR was considered haemodynamically significant at a threshold of 0.80 or less.
CT PERFORMANCE, IMAGE RECONSTRUCTION AND EVALUATION
CT performance and image reconstruction have been described previously7,13. In brief, CT was performed using 64-detector row or higher scanners with prospective or retrospective electrocardiographic gating in accordance with Society of Cardiovascular Computed Tomography guidelines14. All CTs were interpreted in an intention-to-diagnose fashion and analysed within an independent core laboratory by a level III experienced reader blinded to clinical, ICA and FFR results. CT analysis was performed on dedicated 3D workstations (Ziostation; Ziosoft, Redwood City, CA, USA; Advantage Windows (AW) Workstation; GE Healthcare, Milwaukee, WI, USA). CTs were evaluated by an array of post-processing techniques, including axial, multiplanar reformat, maximum-intensity projection, and short-axis cross-sectional views. In each coronary artery segment, coronary atherosclerosis was defined as tissue structures ≥1 mm2 that existed either within the coronary artery lumen or adjacent to the coronary artery lumen that could be discriminated from surrounding pericardial tissue, epicardial fat, or the vessel lumen itself. Lesion length and plaque burden were measured.
Coronary arteries and branches were categorised into one of four vascular territories: left main artery (LM), left anterior descending artery (LAD), left circumflex artery (LCx) and right coronary artery (RCA); diagonal branches, obtuse marginal branches and posterolateral branches were considered as part of the LAD, LCx and RCA system, respectively. The posterior descending artery was considered as part of the RCA or LCx system, depending upon the coronary artery dominance.
APCs –including PR, LAP and SC– were evaluated for coronary lesions directly interrogated by FFR. Stenosis severity was graded in accordance with societal guidelines, and categorised as 0%, 1-29%, 30-49%, 50-69% and ≥70%15. The outer vessel contour was defined as the visualised border at which point low attenuation (epicardial fat) was observed. A remodelling index was defined as a maximal lesion vessel diameter divided by proximal reference vessel diameter, with PR defined as a remodelling index ≥1.1. LAP was defined as any voxel <30 Hounsfield units within a coronary plaque. SC was defined by an intralesion calcific plaque <3 mm in length that comprised <90° of the lesion circumference. Our laboratory’s intraobserver and interobserver reproducibility data for CT imaging have been reported previously3.
Statistical analysis
Continuous variables were compared by the unpaired t-test for normally distributed variables and by the Mann-Whitney U test for skewed variables. Categorical variables were tested by Pearson’s chi-square or Fisher’s exact test as appropriate. The results of continuous variables were presented as mean±SD. FFR and FFRCT measurements were recorded on a continuous scale and dichotomised at a 0.80 threshold, with FFR values ≤0.80 considered haemodynamically significant and causal of ischaemia. High-grade stenosis by CT was dichotomised at the 50% threshold, with stenosis of ≥50% considered obstructive. Global chi-square analyses utilised logistic regression and a likelihood ratio test. To examine discrimination, area under the receiver operating characteristics (AUC) curves were constructed from predicted probabilities of logistic regression models and compared non-parametrically for correlated data16. In order to account for the correlation within patients of coronary artery segments with plaque in an unbalanced design, a random effects model using a maximum likelihood logit model for panel data was applied (STATA xtmelogit command), wherein the binary outcome value of FFR ≤0.80 was modelled using a binomial distribution and a logit link function, and with the individual patient serving as the random component. The random effects model odds ratio estimates were employed to evaluate predictors of ischaemia in univariable and multivariable analyses17. Further, a category-free net reclassification improvement (NRI) of APCs was obtained using the resultant predicted probabilities of the random effects logit models predicting ischaemia where model 1 consisted of CT stenosis ≥50% or <50% plus FFRCT, and model 2 comprised model 1 plus any APC feature, number of APCs, or individual APC18. Category-free NRIs were calculated using an SAS macro19. Statistical tests were two-sided, with a significance level set at 0.05. Statistical analysis was performed using STATA software, Version 12 (StataCorp LP, College Station, TX, USA) and SAS software, Version 9.2 (SAS Institute Inc., NC, USA).
Results
STUDY POPULATION
The study population comprised a total of 252 consecutive patients, for whom 407 coronary lesions were directly interrogated by FFR. The baseline characteristics of the study population are listed in Table 1.
RELATIONSHIP OF ATHEROSCLEROTIC PLAQUE CHARACTERISTICS BY CT TO LESION-SPECIFIC ISCHAEMIA BY FFR
For the 407 lesions, the mean FFR value was 0.82±0.13. Two hundred and fifteen (53%) lesions were obstructive (CT stenosis ≥50%) while 192 (47%) lesions were non-obstructive. Coronary artery lesions which caused ischaemia were of higher stenosis severity by CT when compared to non-ischaemic lesions (CT stenosis grade score: 4.4±1.0 vs. 3.3±1.3, p<0.0001). Mean FFRCT value was lower in obstructive lesions when compared to non-obstructive lesions (0.67±0.15 vs. 0.82±0.11, p<0.0001). Increasing numbers of APCs were observed within ischaemic lesions compared to non-ischaemic lesions, with one, two and three APCs present at a 1.5-fold, fourfold, and sixfold higher prevalence (32% vs. 20%, 35% vs. 9%, 17% vs. 3%, respectively). As compared to lesions that did not cause ischaemia, those that caused ischaemia were associated with a threefold, fivefold and threefold higher prevalence of SC, LAP and PR, respectively (Table 2). Lesion length, plaque burden and LAD lesion were also associated with lesion-specific ischaemia.
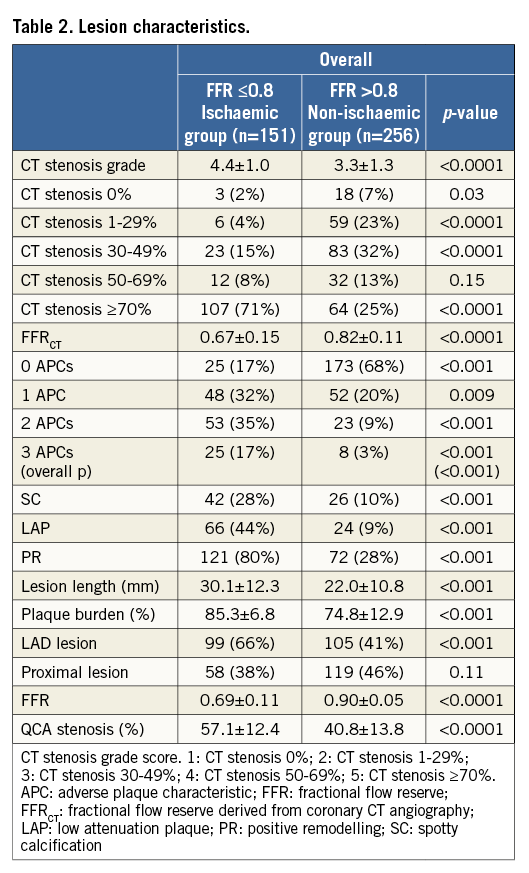
In univariable analysis for detection of lesion-specific ischaemia, CT stenosis ≥50% (odds ratio [OR] 8.7, 95% confidence interval [CI]: 4.2-18.1, p<0.001), FFRCT ≤0.8 (OR 8.6, 95% CI: 4.6-16.0, p<0.001), any APC (OR 11.6, 95% CI: 6.2-21.9, p<0.001) and increasing numbers of APCs (1 APC: OR 6.9, 95% CI: 3.6-13.3, p<0.001; ≥2 APCs: OR 19.7, 95% CI: 9.1-42.5, p<0.001) were associated with a higher prevalence of lesion-specific ischaemia. Individual APCs –SC, LAP, PR– were also associated with ischaemia; lesion length, plaque burden and LAD lesion were also associated with lesion-specific ischaemia (Table 3). In multivariable analyses accounting for obstructive stenosis, FFRCT, plaque burden, LAD lesion and APCs, any APC (OR 7.3, 95% CI: 3.8-13.9, p<0.001) and increasing numbers of APCs (1 APC: OR 4.9, 95% CI: 2.4-9.9, p<0.001; ≥2 APCs: OR 11.2, 95% CI: 5.2-24.5, p<0.001) were associated with ischaemia. Among individual APCs – independent of CT stenosis, FFRCT, plaque burden, LAD lesion and PR but not SC or LAP were associated with a fourfold increased prevalence of lesion-specific ischaemia when compared to coronary plaques that did not possess any APC (Table 3). Incremental risk prediction of APCs beyond obstructive CT stenosis and FFRCT was observed with global chi-square values (Figure 1).
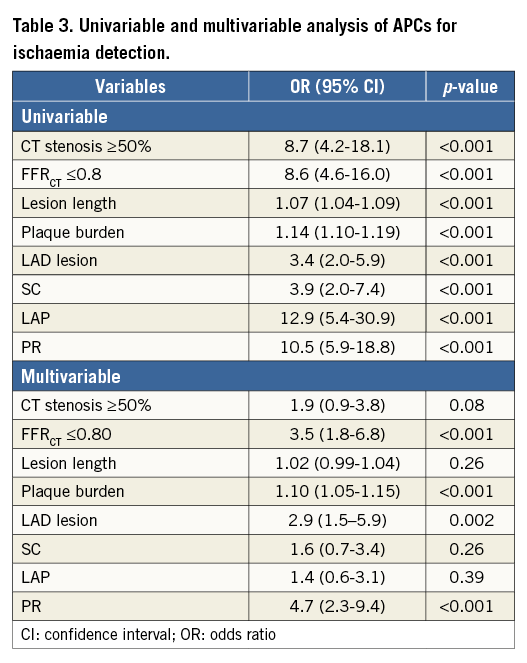
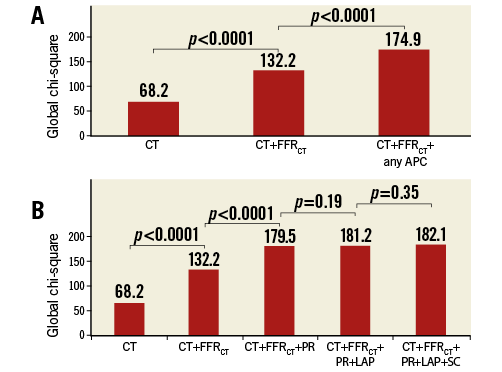
Figure 1. Global chi-square values. Global chi-square values demonstrating incremental risk prediction beyond CT stenosis ≥50% and FFRCT when adding the presence of any adverse plaque characteristics (APC) (A), or positive remodelling (PR) but not for low attenuation plaque (LAP) and spotty calcification (SC) (B). FFRCT: fractional flow reserve derived from coronary CT angiography
RELATIONSHIP OF ADVERSE PLAQUE CHARACTERISTICS TO LESION-SPECIFIC ISCHAEMIA STRATIFIED BY CORONARY ARTERY STENOSIS SEVERITY
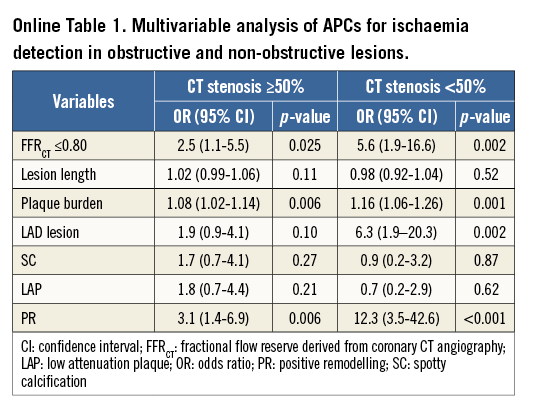
Figure 2 depicts the APC prevalence for ischaemic versus non-ischaemic lesions, as stratified by obstructive versus non-obstructive stenoses. For ≥50% versus <50% stenoses, ischaemia was observed in 119 of 215 (55%) and 32 of 192 (17%) lesions, respectively. Mean FFRCT value was 0.71±0.15 for obstructive lesions and 0.82±0.10 for non-obstructive lesions. PR, LAP, SC and increasing numbers of intraplaque APCs were associated with ischaemia for both anatomically obstructive as well as non-obstructive coronary lesions. In multivariable analyses accounting for FFRCT, lesion length, plaque burden, LAD lesion and all APCs, PR but not SC or LAP was associated with higher odds of ischaemia within a coronary lesion for both obstructive lesions (OR 3.1, 95% CI: 1.4-6.9, p=0.006) and non-obstructive lesions (OR 12.3, 95% CI: 3.5-42.6, p<0.001) (Online Table 1).
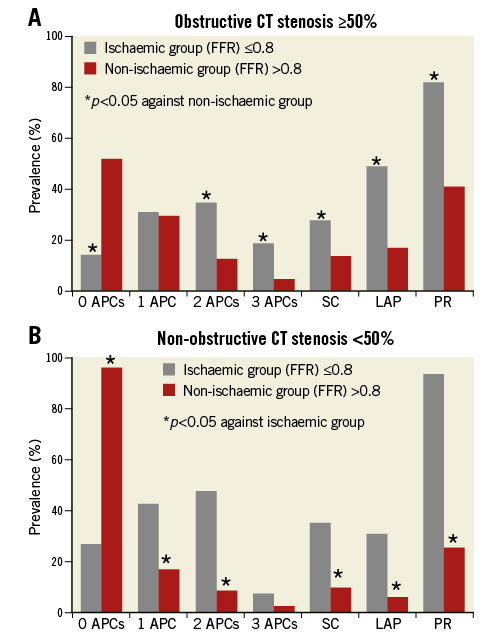
Figure 2. Prevalence of APCs as stratified by stenosis.
DISCRIMINATION OF ISCHAEMIA BY CORONARY CTA
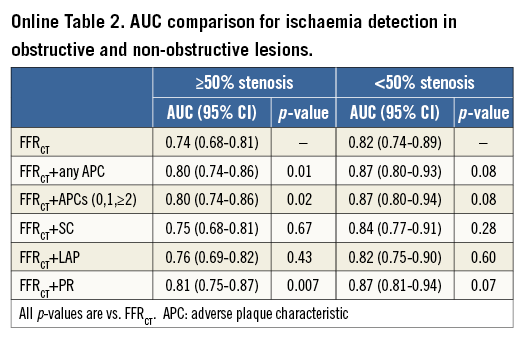
In adjusted logistic regression analyses, FFRCT provided incremental prediction for lesion ischaemia over CT stenosis ≥50% (AUC 0.83 [95% CI: 0.79-0.87] vs. AUC 0.71 [95% CI: 0.66-0.75], p<0.01) (Figure 3). Further, any APC (Figure 3A) or numbers of APCs (Figure 3B) provided incremental prediction for lesion ischaemia over CT stenosis ≥50% plus FFRCT (both AUC 0.87 [95% CI: 0.83-0.90] vs. AUC 0.83 [95% CI: 0.79-0.87], p=0.003). Among individual APCs, PR (AUC 0.87 [95% CI: 0.83-0.90] vs. AUC 0.83 [95% CI: 0.79-0.87], p=0.002) but not SC or LAP provided incremental prediction for lesion ischaemia over CT stenosis ≥50% plus FFRCT (Figure 4).
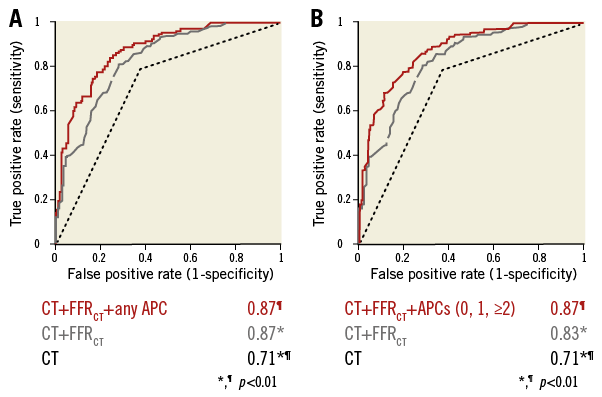
Figure 3. Discrimination of ischaemia by CT. Areas under the receiver operating characteristic curve of APCs (A: any APC, B: numbers of APCs), FFRCT and CT stenosis compared with invasive FFR for diagnosis of ischaemia. FFR: fractional flow reserve
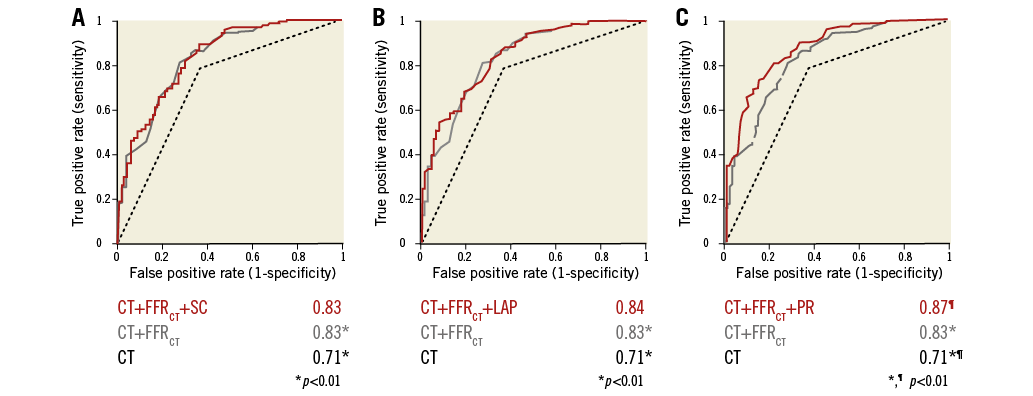
Figure 4. Discrimination of ischaemia by CT by individual APC. Areas under the receiver operating characteristic curve of individual APC (A: SC, B: LAP, C: PR), FFRCT and CT stenosis compared with invasive FFR for diagnosis of ischaemia.
These findings are consistent in obstructive lesions (Online Table 2). In non-obstructive lesions, PR had a trend towards improved incremental prediction of lesion ischaemia (AUC 0.87 [95% CI: 0.81-0.94] vs. AUC 0.82 [95% CI: 0.74-0.8], p=0.07).
NET RECLASSIFICATION IMPROVEMENT OF ISCHAEMIC LESIONS
APCs were associated with improved reclassification of lesions that cause ischaemia over CT stenosis and FFRCT. Among APCs, SC (NRI 0.35, 95% CI: 0.19-0.51, p=0.0006), LAP (NRI 0.91, 95% CI: 0.73-1.09, p<0.0001) and PR (NRI 0.98, 95% CI: 0.81-1.15, p<0.0001) enabled effective reclassification of ischaemic lesions for CT stenoses ≥50% and FFRCT.
An example of an obstructive non-ischaemia-causing coronary artery lesion that does not possess APCs and negative FFRCT is depicted in Figure 5. An example of an obstructive ischaemia-causing coronary artery lesion possessing APCs and positive FFRCT is depicted in Figure 6.
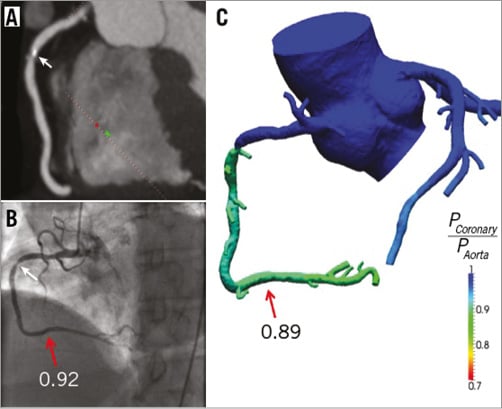
Figure 5. Representative example of lesion with non-ischaemic RCA obstructive stenosis. A) Multiplanar reformat of CT demonstrating obstructive stenosis (white arrow) with no APCs (PR [–], LAP [–], SC [–]) in the proximal portion of RCA. B) Invasive coronary angiogram demonstrates obstructive stenosis (white arrow) and FFR value of 0.92 (red arrow), indicating vessel no-ischaemia. C) FFRCT value of 0.89 (red arrow) indicating vessel no-ischaemia. QCA: quantitative coronary angiography; RCA: right coronary artery
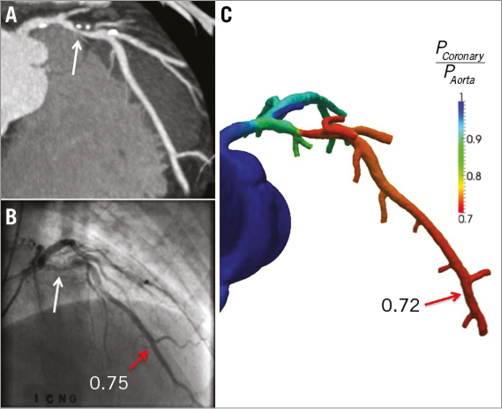
Figure 6. Representative example of a lesion with ischaemic LAD obstructive stenosis. A) Multiplanar reformat of CT demonstrating obstructive stenosis (white arrow) with APCs (PR [+], LAP [+], SC [+]) in the proximal portion of LAD. B) Invasive coronary angiogram demonstrates obstructive stenosis (white arrow) and FFR value of 0.75 (red arrow), indicating vessel ischaemia. C) FFRCT value of 0.72 (red arrow) indicating vessel ischaemia. LAD: left anterior descending artery
Discussion
In this prospective international multicentre study, we identified a strong relationship between APCs by CT and ischaemia-causing coronary lesions by FFR. We observed an increasing prevalence of APCs within coronary lesions of higher stenosis severity. However, independent of stenosis severity, APCs within coronary lesions were associated with heightened prevalence of ischaemia, with increasing numbers of intralesion APCs demonstrating a dose-response relationship towards ischaemia risk. We further noted a strong relationship between APCs and lesion-specific ischaemia, even amongst non-obstructive lesions that do not meet conventional thresholds of being angiographically severe. Similarly, the absence of APCs identified lesions with a considerably lower prevalence of ischaemia, even for highly stenotic coronary lesions. Finally, among individual APCs, PR was strongly associated with lesion-specific ischaemia and provided incremental prediction for lesion-specific ischaemia over CT stenosis plus FFRCT. SC and LAP are less strongly associated with lesion-specific ischaemia.
Recent technological developments have advanced the feasibility of CT for physiologic evaluation by applying computational fluid dynamics for non-invasive calculation of FFR from typically acquired CT (FFRCT). This technique demonstrates significantly improved diagnosis of coronary lesion-specific ischaemia in a manner superior and additive to CT stenosis severity alone7,8. In a previous study, FFRCT demonstrated greater discrimination over CT stenosis alone for the diagnosis of lesions which cause ischaemia7,8. In the current study, we have demonstrated that APCs improve discrimination and reclassification of ischaemia-causing lesions incremental to both CT stenosis severity and FFRCT. To our knowledge, these data represent the first to examine the relationship of CT APCs to identify precisely coronary artery lesions that do versus those that do not cause ischaemia over CT stenosis and FFRCT. Given the use of an invasive FFR reference standard, the large number of coronary lesions interrogated by blinded independent core laboratories and the multicentre international nature of the study, these results should be considered widely generalisable in scope.
In the present study, we identified a distinct relationship between APCs and ischaemia, independent of stenosis severity and FFRCT. Specifically, PR was associated with higher rates of ischaemia-causing lesions in both obstructive and non-obstructive stenoses. While the mechanism for this finding cannot be fully realised in the present study, a number of potential reasons may explain this finding. First, the development of PR may represent a point in the cycle of an atheroma wherein the overall plaque burden is sufficiently high to trigger compensatory remodelling, and that this stage is associated with lesion ischaemia. Further, it is feasible that the presence of PR (or other APCs) is an anatomic surrogate of local physiologic processes, such as endothelial dysfunction or inflammation, and that these findings are associated with lesion-specific ischaemia. Lending support to the former of these explanations are the findings of the FIRST study by Waksman et al, wherein FFR correlated only with plaque burden, but not with other morphologic plaque features assessed by invasive imaging20. Importantly, the FIRST study evaluated only intermediate stenosis lesions, and supports the strength of thresholds of plaque burden as a marker of ischaemia.
To date, the preponderance of studies evaluating APCs by CT have been related to ACS rather than to ischaemia4,5,21,22. These studies suggest a correlation between APCs and ACS event risk, with APCs by CT –particularly PR– associated with future acute rather than stable events. In PROSPECT, the landmark study of individuals who had experienced ACS with uncomplicated coronary revascularisation and prospectively underwent virtual histology IVUS (VH-IVUS)23, numerous plaque features –such as plaque burden, minimum luminal area and thin-cap fibroatheroma (TCFA)– were associated with incident ACS events, findings corroborating both CT as well as histopathologic studies. Nevertheless, the relationship of these invasive findings to CT APCs remains unknown. To date, the precise mechanisms of why these features are important in the ACS process are poorly understood, but it may be that the morphologic attributes of a plaque are associated with ischaemia, the latter of which may represent a poor prognostic indicator for adverse clinical events in a lesion-specific fashion.
At present, clinical interpretation of CT is based primarily on stenosis severity, a method advocated by societal guidance documents15. Yet, in the present study, APCs enabled effective reclassification of lesions that did versus those that did not cause ischaemia over measures of stenosis severity alone, as well as FFRCT. These results reinforce a concept that the evaluation of luminal narrowing alone by CT is inadequate to identify specific coronary lesions that are ischaemia-causing12.
Study limitations This study is not without limitations. Importantly, while an array of prior studies has observed CT assessment of APCs to be concordant with invasive evaluation by IVUS, current-generation CT is limited in spatial and temporal resolution, and thus cannot identify TCFA, a plaque feature that is vital to the coronary disease process. While it remains unknown whether TCFA is directly related to the ischaemic process, the APCs noted by CT in the present study are directly associated with TCFA when compared to optical coherence tomography, and may help to explain further our current study findings24. Further, the coronary vessels interrogated by FFR were limited to those deemed clinically indicated. In this regard, a potential bias of selection and/or referral cannot be discounted from the present study results. In addition, it is possible that the presence of atherosclerosis in other major epicardial coronary vessels may have influenced the presence or absence of ischaemia in the FFR-interrogated vessel, and this premise could not be adequately tested given the lack of FFR in non-interrogated vessels. Finally, at present, only one commercial company possesses a computational fluid dynamic solution for the calculation of FFRCT, with several other groups developing their own proprietary software. The workflow of how FFRCT will be utilised in clinical practice –whether on or off site– remains to be determined, as this technology is still in its investigational stage and not approved for commercial use in the USA.
Conclusions
Independent and incremental to stenosis severity and FFRCT, atherosclerotic plaque features –especially PR– by CT improve identification and reclassification of specific coronary artery lesions that cause ischaemia.
| Impact on daily practice CT enables assessment of several coronary atherosclerotic plaque characteristics (APCs). Recently, fractional flow reserve derived from CT (FFRCT) has emerged as a novel non-invasive method that demonstrates high diagnostic performance for identification and exclusion of ischaemia. APCs are associated with ischaemia and improve identification of ischaemia over CT stenosis and FFRCT. |
Funding
This study was funded by HeartFlow, Inc., Redwood City, CA, USA. HeartFlow, Inc., did not have involvement in the design of the study, nor were they involved in the data analysis, manuscript preparation, and review or authorisation for submission. This study was also funded, in part, by a generous gift from the Dalio Institute of Cardiovascular Imaging and the Michael Wolk Foundation. The research reported in this publication was supported by the Heart Lung and Blood Institute of the National Institutes of Health under award number R01 HL115150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement
J. Min serves as a consultant to HeartFlow, Inc., and serves on the medical advisory board for GE Healthcare. J. Leipsic and M. Budoff serve on the medical advisory board for GE Healthcare. The other authors have no conflicts of interest to declare.
Supplementary data