
In a seminal experimental work almost 50 years ago, Gould and Lipscomb described the haemodynamic consequences of progressive focal reductions in coronary artery diameter on coronary flow1. It was observed that stenosis ≥50% and ≥85% of the lumen diameter impairs maximal hyperaemic and baseline flow, respectively. A sine qua non relationship between coronary stenosis, myocardial ischaemia and adverse cardiovascular events matured, and slowly became the reigning paradigm of ischaemic heart disease (IHD). In contrast with this “stenosis-centred” theory of IHD, Likoff et al described a group of women with angina, ischaemic electrocardiographic responses to physical exercise, and normal coronary angiograms in 19672. Numerous studies then reported that these patients, likely suffering from ischaemia and non-obstructive coronary arteries (INOCA), have a poorer long-term prognosis3. Diffuse coronary atherosclerosis (DCA) also deserves a historical note. Post-mortem series described the diffuse nature of atherosclerosis 50 years ago4. It was not, however, from the field of ischaemia that DCA achieved significant consideration. Rather it was from the description in 1989 by Muller et al of either severe fixed stenosis or even only “luminal irregularities” with a higher propensity to rupture and cause myocardial infarctions than vulnerable plaques4. Nearly 10 years later, it was demonstrated that DCA produces a gradual base-to-apex, longitudinal perfusion gradient, compatible with fluid dynamic theory, and with the graded, continuous pressure loss along the arterial length observed by De Bruyne5.
In the catheterisation laboratory, fractional flow reserve (FFR) has become the standard method to assess obstructive IHD following the demonstration that physiological rather than anatomical revascularisation results in better patient outcomes. Nonetheless, FFR is a lone pressure index and, although coronary pressure and flow are closely interconnected, it has been recognised that flow is primarily more important than pressure for the preservation of myocardial function5. Coronary flow reserve (CFR) is the physiology index that summarises flow. CFR reflects the capacity of both the epicardial vessel and microcirculation to increase flow to satisfy demand. CFR has been thoroughly investigated, and a substantial risk for cardiovascular morbidity and mortality has been observed when it is exhausted3. Measuring CFR in the human beating vessel, however, is technically demanding and not free from error, mostly due to a reported relative inconsistency in the resting status and hyperaemic response3,6.
Coronary flow capacity (CFC) is a two-dimensional concept that integrates CFR with hyperaemic flow within the same physiology metric6. It was originally derived from positron emission tomography (PET) data and proposed as a solution to some of the above-mentioned limitations of CFR. For example, in patients with normal coronary circulations experiencing anxiety or uncontrolled hypertension (leading to an increased rest myocardial workload), baseline flow may be high, while hyperaemic flow is adequate to satisfy demand. Here, ischaemia is absent, but CFR may be low. Conversely, in patients with IHD treated medically, hyperaemic flow may be reduced and, due to optimal medical therapy, baseline flow can be low. This combination may result in a normal CFR6. CFC overcomes this CFR limitation by incorporating absolute hyperaemic flow. Importantly, PET is not capable of discriminating whether flow impediment arises from the epicardial vessel or the microcirculation. Intracoronary guidewires fitted with pressure and flow sensors are the only tools capable of adding this indispensable further layer of information. CFC has already been translated to the invasive field in two reports3,7. The first one investigated the prognostic role of CFC after PCI deferral in patients investigated with Doppler wires. The second one, which preceded the work herein discussed, reported the prognostic value of thermodilution-derived CFC in patients in whom revascularisation was either performed or deferred. Remarkably, in both studies, CFC emerged as a more powerful predictor of future adverse events when compared with CFR and FFR.
In this issue of EuroIntervention, Hoshino et al further expand their previous findings by focusing only on the prognostic role of CFC in patients where revascularisation was deferred on the basis of FFR8.
Initially, the authors investigated 687 de novo intermediate lesions with pressure and thermodilution-sensitive wires. From these, 308 lesions from 308 patients were deferred and included in this work. This deferred population is extremely important because the most significant merit of coronary physiology is its capacity to discriminate vessels and patients at low risk when left to medical treatment alone. At 30 months, only 2 cardiac deaths (0.64%), 2 non-fatal myocardial infarctions (0.64%) and 15 vessel-oriented revascularisations (4.87%) occurred. Hence, the overall rate of vessel-oriented cardiovascular events (VOCE) at 2.5 years amounted to only 6.2%. This rate is relatively low, and lower than that observed for most of the coronary stents that we currently use. An important first message of this work is thus reassurance on the safety of revascularisation deferral based on FFR. Afterwards, the authors observed that only CFC and FFR were independent predictors of VOCE, and only age, prior PCI and CFC were independent predictors of major adverse cardiac events (MACE, defined as VOCE, non-target vessel revascularisation or heart failure). Finally, CFC exhibited an incremental capacity to discriminate and reclassify both VOCE and MACE when incorporated into models with clinical variables and FFR or CFR. Altogether, the present study suggests that, from all available flow-based indices obtained with thermodilution-sensitive wires, including CFR, CFC and index of microcirculatory resistance, CFC is the one with the highest capacity to discriminate stenosed vessels at higher risk for MACE.
Some important limitations are worth discussing. First, categorisation of invasive CFC has been based on percentiles and not on clinical or pathophysiological cut-offs. Second, the use of the inverse of mean transit time as a lone physiology index carries intrinsic limitations. Being an absolute index, transit time depends on the size of the perfused myocardial territory. It is currently unclear to what extent this theoretical concern limits CFC clinical applicability. The low rate of events, its definition, and the modest sample size should also be acknowledged.
Clear clinical messages that arise from invasive CFC studies are: 1) not all FFR-negative vessels carry the same risk, and 2) flow-sensitive wires allow further identification of this concealed risk. A proposed reinterpretation of the CFR and FFR relationship suggests that significant degrees of DCA or microvascular disease, as discussed above, could exhaust the CFR without significantly affecting FFR5. It seems unlikely that these vessels, identified better by CFC, will receive significant benefit from revascularisation, because the focal component of IHD, resolvable with stents, is minor. This information, in conjunction with post-PCI physiology, pressure pullbacks and co-registration, is slowly revealing a subgroup of vessels where DCA or INOCA is significant, associated with higher risks, and hence frustrating to treat (Figure 1). The current report from Hoshino serves as a lesson in humility. The first step in solving any problem, however, is recognising that there is one. We can now recognise FFR-negative vessels at higher risk. This paves the way for studies evaluating their treatment. Just because the road ahead is long, there is no reason to slow down. It is the reason to get started.
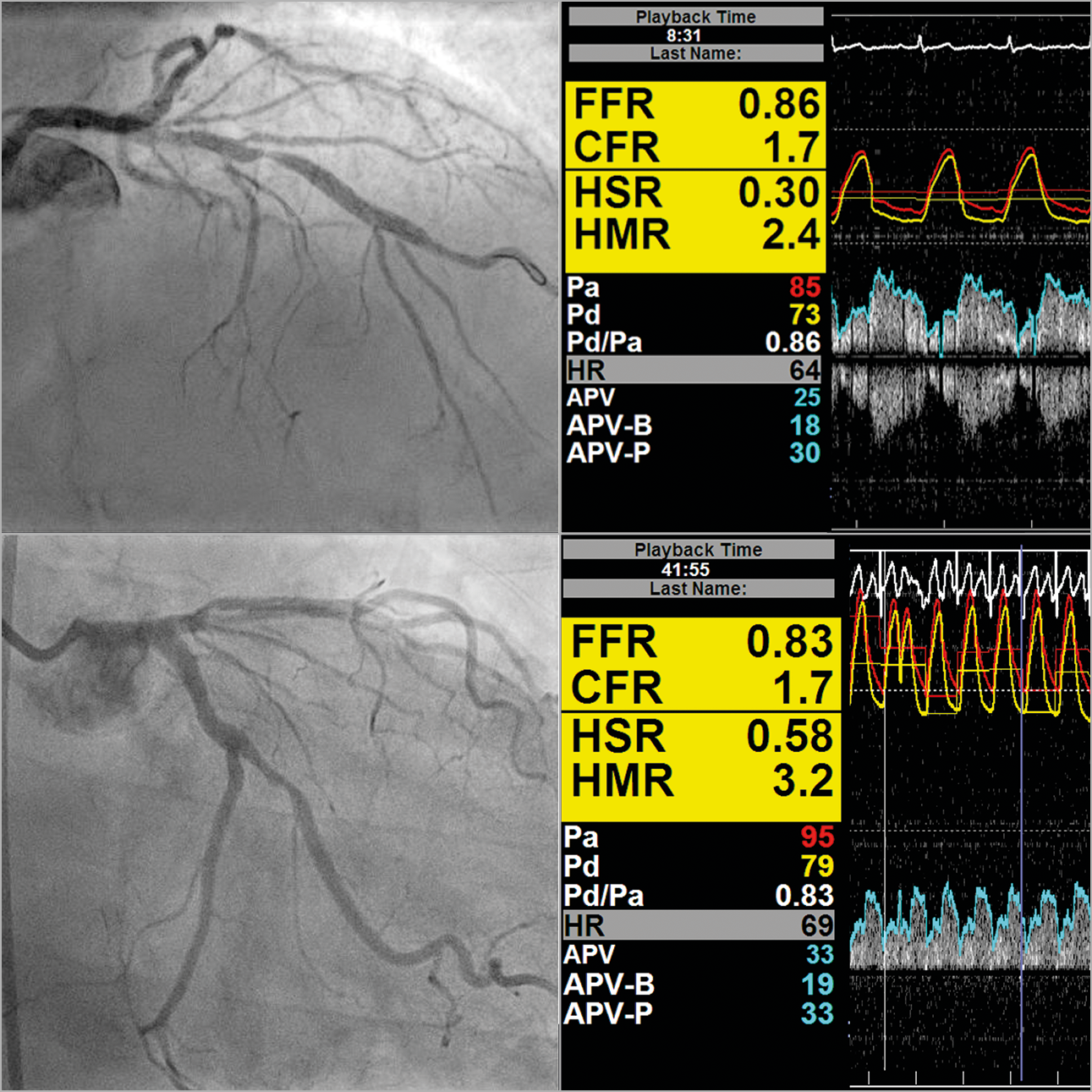
Figure 1. A 67-year-old patient with stable angina, CCS 2. Diffuse and focal atherosclerosis is evident. FFR is negative in the left system. CFC, however, is moderately exhausted, suggesting a higher risk.
Conflict of interest statement
M. Echavarria-Pinto and T. van de Hoef have served as speakers in educational events organised by Abbott and Philips.
Supplementary data
To read the full content of this article, please download the PDF.

