Abstract
Background: Coronary flow capacity (CFC) provides integrated information about coronary flow reserve (CFR) and hyperaemic coronary flow and is useful for identifying coronary flow limitation.
Aims: The aim of this study was to investigate the effect of percutaneous coronary intervention (PCI) on vessel-related major adverse cardiovascular events (MACE) according to CFC status in stable coronary lesions.
Methods: From a global, multicentre registry of comprehensive physiological assessment, a total of 1,397 patients (1,694 vessels) were analysed. Low CFC was defined for lesions with reduced CFR and inverse of hyperaemic mean transit time (1/hTmn). A predefined definition of CFC (CFR <2.0 and 1/hTmn less than the corresponding percentile) was assessed first in a multivariable marginal Cox proportional model with the interaction term between CFC status and PCI (performed or not), and then the optimal definition of CFC was explored.
Results: We observed a significant interaction between predefined low CFC and PCI (p=0.067). With the optimal definition of CFC (CFR ≤3.2 and 1/hTmn ≤2.8), the HR (95% CI) of PCI was 0.278 (0.103-0.751) and 1.393 (0.783-2.478) in lesions with low and normal CFC, respectively. If lesions with fractional flow reserve (FFR) ≤0.8 and normal CFC had been deferred, the number of PCI would have decreased by 64%.
Conclusions: FFR-guided PCI for low CFC lesions was associated with reduced incidence of MACE in low CFC but not in normal CFC lesions. Our results suggest the potential use of CFC in combination with FFR for optimising the indication for PCI by reducing potentially unbeneficial PCI. Clinical Trials Registration: https://clinicaltrials.gov/ct2/show/NCT03690713
Introduction
Large-scale prospective studies have not consistently demonstrated a prognostic benefit of elective percutaneous coronary intervention (PCI) to stable lesions1,2. A recent meta-analysis demonstrated that fractional flow reserve (FFR)-guided PCI could reduce the composite events of cardiac death or myocardial infarction (MI)3. In practice, FFR is the current standard for determining the severity of epicardial coronary functional stenosis and indication for PCI. Recently, some studies have reported the identification of subsets of patients who might effectively benefit from PCI, not by using FFR but by using other indices4,5. Since PCI is invasive with associated procedural costs for healthcare systems, more focused selection of patients who would benefit from elective PCI in terms of prognosis warrants further investigation.
Coronary flow capacity (CFC) is a relatively novel concept in which coronary flow reserve (CFR) and hyperaemic coronary flow are integrated to capture comprehensive flow characteristics4,6,7. CFC was first developed using non-invasive positron emission tomography (PET) and was subsequently verified using invasive intracoronary wires. CFC by definition reflects the status of flow limitation and might provide useful prognostic information for both deferred and revascularised stable lesions that could not be captured solely by CFR or FFR4,7. However, to date, the difference in the prognostic benefit of FFR-guided PCI according to CFC or other physiological parameters has not been determined.
We hypothesised that the consideration of CFC combined with FFR would lead to better selection of stable lesions in terms of PCI benefit compared with current practice (i.e., FFR guidance for revascularisation). This global, multicentre collaboration study aimed to investigate the prognostic effect of PCI according to CFC status.
Methods
PATIENT POPULATION
The present study was a patient-level pooled analysis of three prospective registries4,8,9. These registries were from Tsuchiura Kyodo General Hospital (Ibaraki, Japan), five university hospitals in South Korea, and the Hospital Clinico San Carlos (Madrid, Spain). All the enrolled patients underwent coronary angiography and the measurement of FFR, resting/hyperaemic mean transit time (rTmn/hTmn), CFR and index of microvascular resistance (IMR) calculation using the PressureWire™ (Abbott Vascular, St. Paul, MN, USA) (Supplementary Appendix 1). In all studies, patients with haemodynamic instability, left ventricular dysfunction, or a culprit vessel of acute coronary syndrome were excluded. Standardised definitions were used for all the variables, and invasively obtained physiologic indices were crosschecked and confirmed by each study’s principal investigators. From a total of 1,694 vessels with stable lesions (1,397 patients), four patients were lost to follow-up or had missing follow-up data. Finally, 1,690 vessels (1,393 patients) were analysed in the present study. Each study’s protocol was approved by the institutional review board or ethics committee at each participating centre and all patients provided written informed consent. The study protocols were in accordance with the Declaration of Helsinki. The study protocol of the International Collaboration of Comprehensive Physiologic Assessment trial was registered in -clinicaltrials.gov (NCT03690713).
The primary outcome, vessel-related major adverse cardiovascular events (MACE), was defined as the composite of death from cardiac causes, target vessel-related MI and target vessel-related ischaemia-driven revascularisation (Supplementary Appendix 1),8,10.
CFC CATEGORISATION
According to the CFC concept derived from previous studies, the CFC categorisations were based on both CFR and the inverse of hTmn4,7. The present study focused mainly on low CFC, which was defined as lesions with both reduced CFR and 1/hTmn (dichotomous variable). Since the cut-offs of CFR and 1/hTmn have not been established for defining CFC, we took the following approach: first, we defined a low CFC status for the vessels with a well described threshold of CFR <2.0 and 1/hTmn less than the matched percentile corresponding to CFR=2.0 (3.03). Then the significance of the interaction between CFR (lower than 2.0 or higher) and PCI (performed or not) and between CFC (low or normal) and PCI for MACE was examined. Second, we explored the optimal thresholds of CFR and 1/hTmn for defining CFC to maximise the statistical efficiency of the Cox model with the interaction term. In detail, 1) low CFC was defined as vessels with CFR ≤x and 1/hTmn ≤y for a given pair of x and y (for example, x=2.0 and y=3.0); 2) the Akaike Information Criterion (AIC) of the model was derived (the lower the better); 3) AIC reduction from the model without the interaction term was calculated, highlighting the model performance (the higher the better); and 4) AIC reductions were compared among models based on various pairs of x and y (within the interquartile range of CFR and 1/hTmn). The optimal definition of CFC was made according to the model with the highest AIC reduction.
STATISTICAL ANALYSIS
Data were analysed on a per-patient basis for clinical characteristics and on a per-vessel basis for comparison of lesion characteristics including physiological studies, and vessel-related clinical outcomes. Categorical data, expressed as frequencies and percentages, were compared using the χ2 test or Fisher’s exact test, as appropriate. Continuous biochemical or physiological data were analysed using the Mann-Whitney test or t-test. Marginal Cox proportional hazards regression models were used to evaluate a per-vessel comparison of MACE incidence. Elective PCI (performed or not), CFC (low or normal), an interaction term between PCI and CFC, and factors showing p<0.10 in the univariate analyses were incorporated into the multivariable model. Two marginal logistic regression models were built for vessel-related MACE using the same variables or same variables without an interaction term. The areas under the curves (AUCs) of these models, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) by comparing these models were calculated. Kaplan-Meier curves and the log-rank test were used to compare survival from MACE in vessels with normal or low CFC stratified according to the treatment by elective PCI. R 3.4.4 (The R Foundation for Statistical Computing, Vienna, Austria) was used for the statistical analyses. The significance was determined using a two-sided p<0.10 for interaction11 and p<0.05 for other analyses.
Results
CFC MAPPING
Median FFR was 0.84 (0.77-0.91) and PCI was performed in 31.9% of patients (539 lesions) in this registry. Figure 1 shows a scatter plot of all vessels based on the CFR and the inverse of hTmn. Low CFC was defined as low CFR as well as low 1/hTmn. Vessels with low CFC were more likely to have an FFR ≤0.8 compared to those with normal CFC, implying that FFR would be an important confounder in the association between CFC status and clinical outcomes.
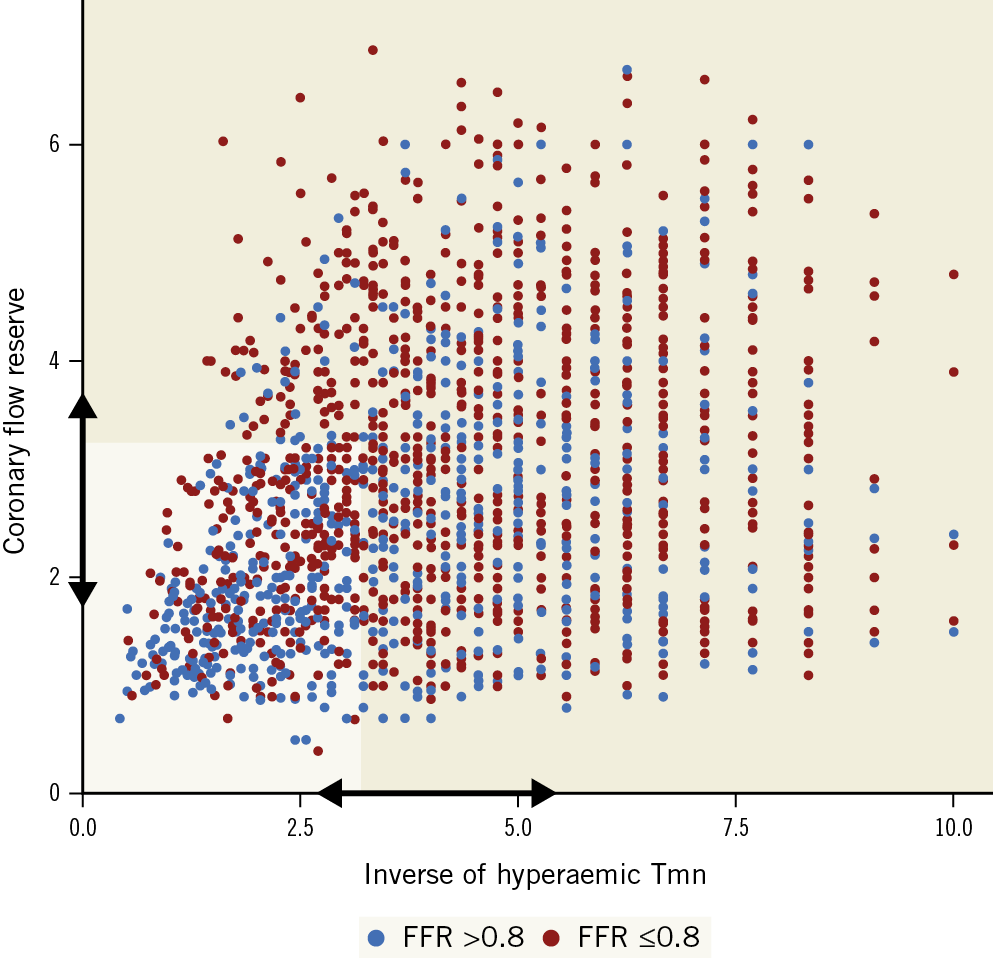
Figure 1. CFC map. Scatter plot showing the distribution of 1,690 vessels by the inverse of mean transit time at hyperaemia (hTmn) representing coronary flow (X-axis) and coronary flow reserve (CFR) (Y-axis). Low coronary flow capacity (CFC) was defined as lesions with low CFR as well as low 1/hTmn. The optimal thresholds of CFR and 1/hTmn were determined according to the performance of multivariable Cox proportional models predicting vessel-related major adverse cardiovascular events (MACE). Interquartile ranges of CFR (1.8 to 3.8) and 1/hTmn (2.6 to 5.6) were tested (arrows indicate these ranges), resulting in CFR ≤3.2 and 1/hTmn ≤2.8 as the optimal thresholds for low CFC (pale area). Vessels with FFR >0.8 and ≤0.8 are shown as blue and red dots.
COMPARISON OF CFR AND CFC FOR PCI GUIDANCE
First, we considered low CFR as CFR <2.0 and low CFC as CFR <2.0 and 1/hTmn <3.03 (corresponding percentile with CFR: 32nd percentile). Two multivariable Cox proportional hazard models were built using the interaction between PCI and CFR (Supplementary Table 1) or PCI and CFC (Supplementary Table 2). The effect of PCI on MACE was different according to CFC status, not CFR status, at the threshold of alpha=0.10 (p=0.067 and 0.78, respectively).
DEFINING THE OPTIMAL DEFINITION OF CFC
Since the thresholds of CFR and 1/hTmn have not been determined for CFC definition, different cut-offs of these indices were tested to derive an optimal definition of CFC for guiding PCI indication (Figure 1, arrows indicate the range tested). A three-dimensional scatter plot showing AIC reduction by different thresholds of CFR and 1/hTmn is shown in Supplementary Figure 1. A model built with the threshold of CFR=3.2 and 1/hTmn=2.8 had the highest AIC reduction of 7.6, indicating the best statistical efficiency of the model. Accordingly, the optimal definition of low CFC was set as vessels with CFR ≤3.2 and 1/hTmn ≤2.8. This definition was used in the following analyses.
CLINICAL FEATURES, OUTCOMES, AND THE INTERACTION BETWEEN CFC AND PCI
Table 1 summarises the population characteristics according to CFC status. Mean age was 64.1±10.3 years and 77.5% (1,080 patients) were male. Low CFC lesions were characterised by tighter diameter stenosis, longer lesion length, shorter 1/rTmn and 1/hTmn, lower FFR and CFR, and higher frequency of being treated by elective PCI.
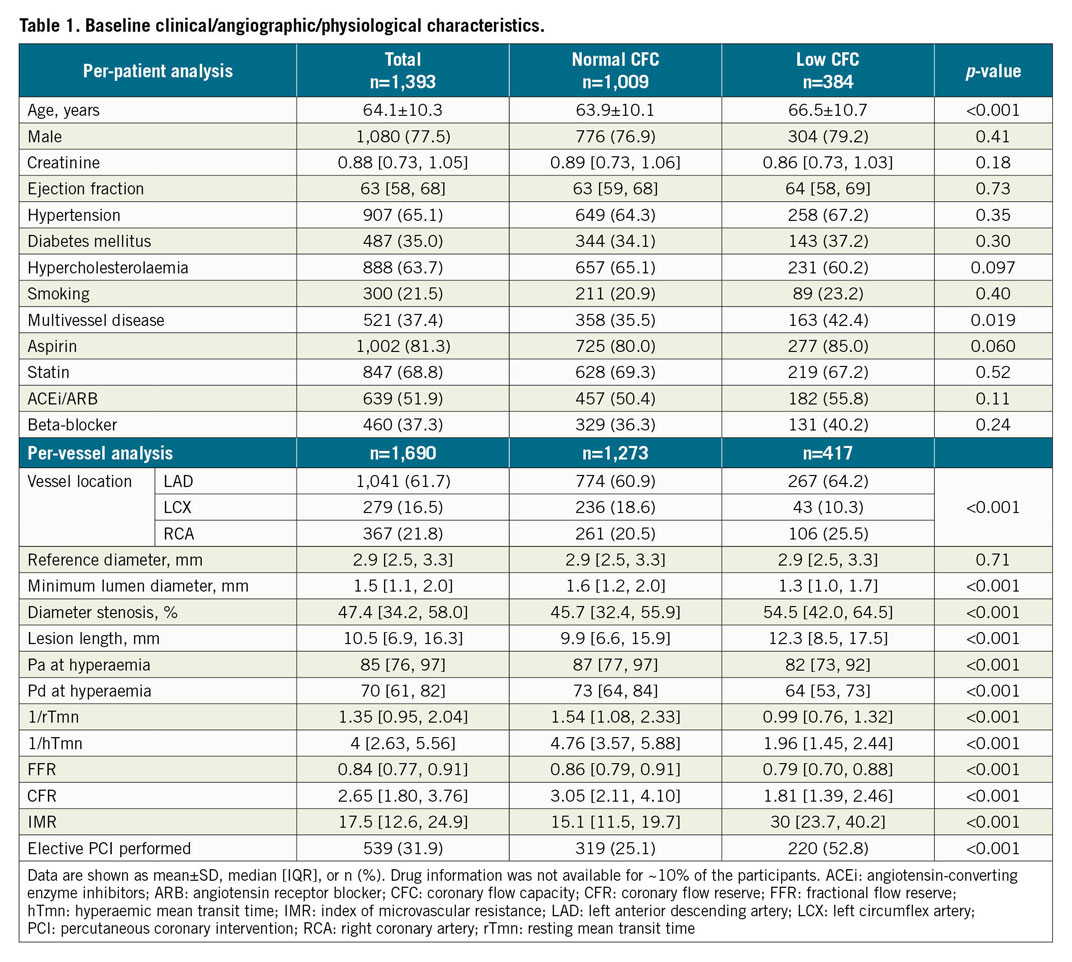
Among vessels with low CFC, MACE occurred in 4.5% (10/220) in five years if treated by PCI and 9.1% (18/197) if not treated by PCI. Among those with normal CFC, MACE was observed in 10.3% (33/319) of vessels treated by PCI and 5.5% (52/954) of vessels not treated. Figure 2 shows Kaplan-Meier curves visualising the survival from MACE in vessels according to CFC status stratified by PCI treatment. Among PCI-treated vessels, the incidence of MACE was significantly lower in lesions with low CFC compared to those with normal CFC (p=0.02). Among deferred vessels, low CFC was significantly associated with higher survival from MACE (p=0.03).
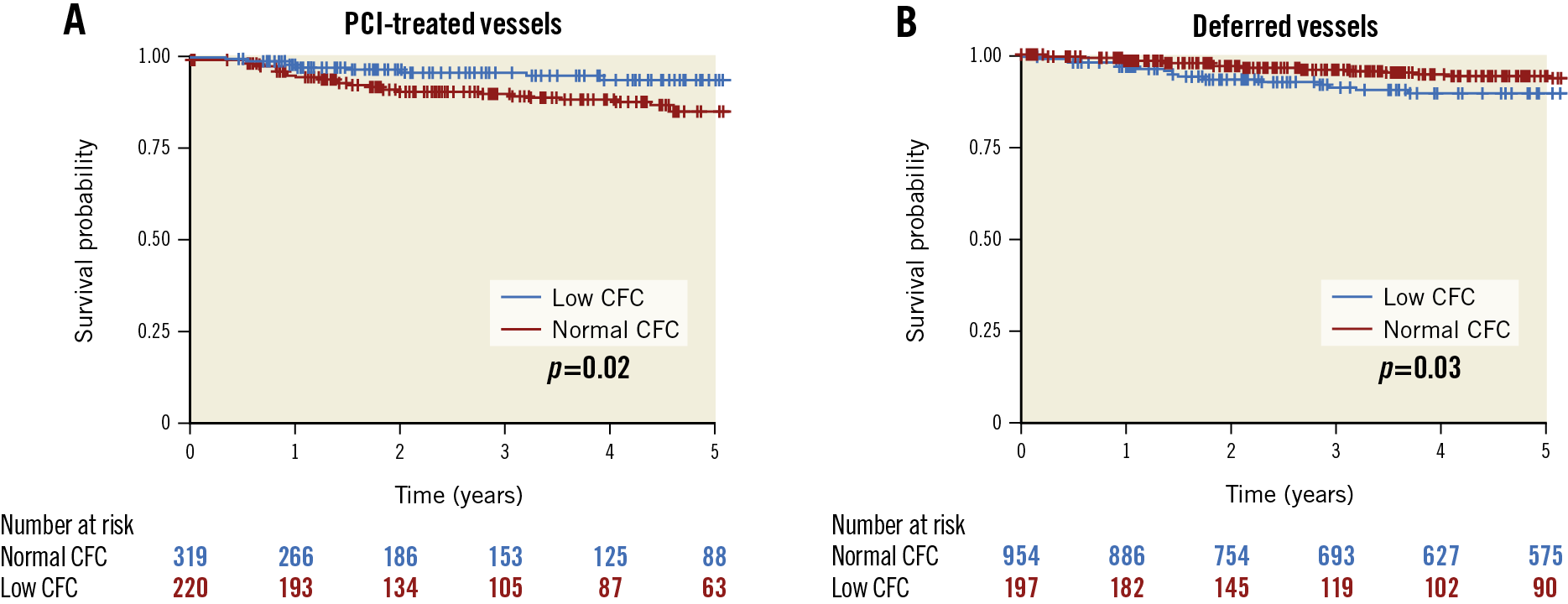
Figure 2. Survival from vessel-related major adverse cardiovascular events (MACE) according to CFC status. Kaplan-Meier curves showing survival from MACE comparing vessels with low CFC (CFR ≤3.2 and 1/hTmn ≤2.8) versus normal CFC. The comparisons are illustrated among PCI-treated vessels (A) and deferred vessels (B). P-values were based on the log-rank test.
In the multivariable Cox model, the interaction between PCI and CFC status was highly significant (p<0.001) (Supplementary Table 3). The hazard ratio (HR) of PCI to lesions with low CFC was 0.278 (95% confidence interval [CI]: 0.103-0.751), suggesting the prognostic benefit of PCI for these lesions. The HR of PCI to lesions with normal CFC was 1.393 (95% CI: 0.783-2.478), which was not significant but implied plausible harmful effects of PCI in this subset.
Based on these findings, we summarised the potential utility of CFC and FFR in guiding elective PCI in Figure 3. By avoiding potentially meaningless PCI to normal CFC lesions, 64% of total PCIs could be deferred compared to FFR-only guidance.
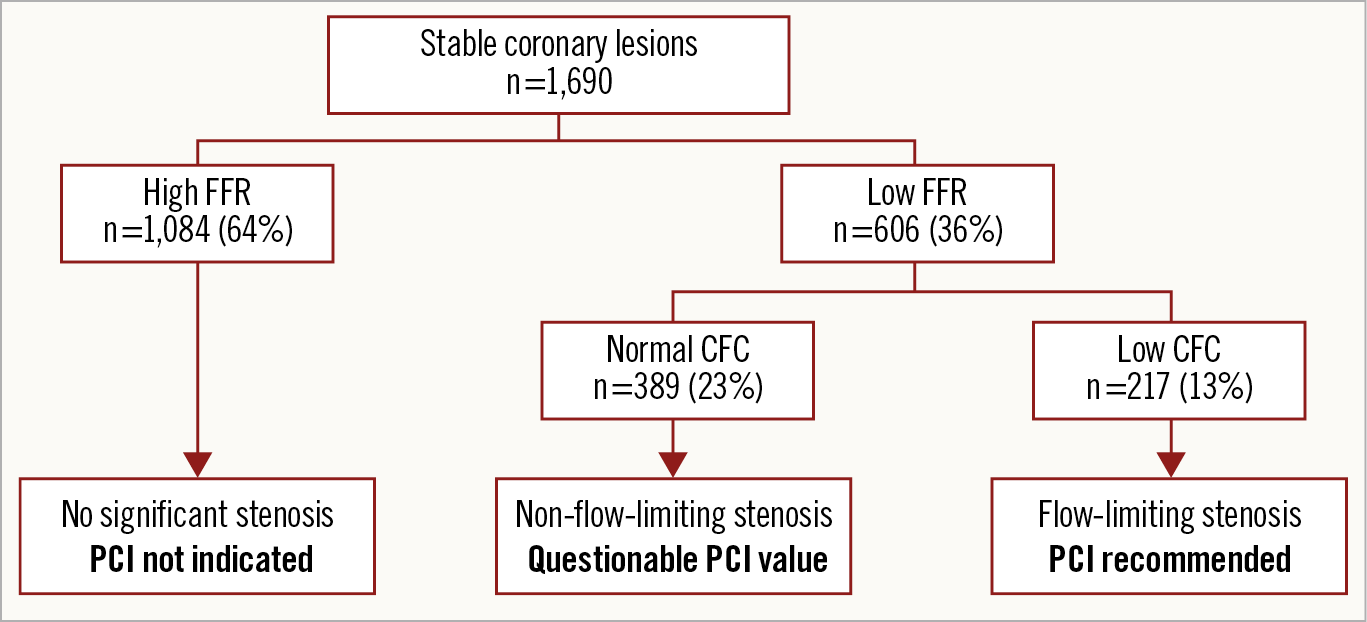
Figure 3. Proposed PCI strategy using FFR and CFC. Sixty-four percent of the vessels indicated for PCI by FFR could be deferred on the basis of CFC status, because PCI to such vessels could increase the risk of future events. FFR-defined epicardial stenosis and CFC-defined flow limitation can provide complementary information regarding the optimisation of PCI indication to stable lesions.
INCREMENTAL VALUE OF CFC STATUS IN PREDICTING MACE
A logistic model consisting of age, diabetes mellitus, hypercholesterolaemia, multivessel disease, FFR, and PCI was built to predict the five-year MACE incidence. The AUC of the model was 0.64 (95% CI: 0.59–0.69). When CFC status and the PCI-CFC interaction term were added to the model, the AUC became 0.67 (95% CI: 0.62–0.72). There was a significant improvement in the discrimination of MACE assessed by NRI (estimate: 0.379, 95% CI: 0.189-0.569, p<0.001) and IDI (estimate: 0.009, 95% CI: 0.003-0.015, p=0.002).
PATHOPHYSIOLOGICAL FEATURES OF CFC
Figure 4 illustrates pathophysiological features of CFC among vessels categorised into four groups: 1) low CFR and low 1/hTmn (low CFC), 2) low CFR and normal 1/hTmn, 3) normal CFR and low 1/hTmn, and 4) normal CFR and normal 1/hTmn (coloured according to these groups). The low CFC group had either low FFR or high IMR or both, in contrast to vessels with low CFR and normal 1/hTmn which had low FFR and non-high IMR, and those with normal CFR and low 1/hTmn which had normal FFR and high IMR. The result suggests that low CFC might imply flow-limiting status due to either microvascular or epicardial dysfunction, which thus could be a reasonable index for PCI indication if combined with FFR.
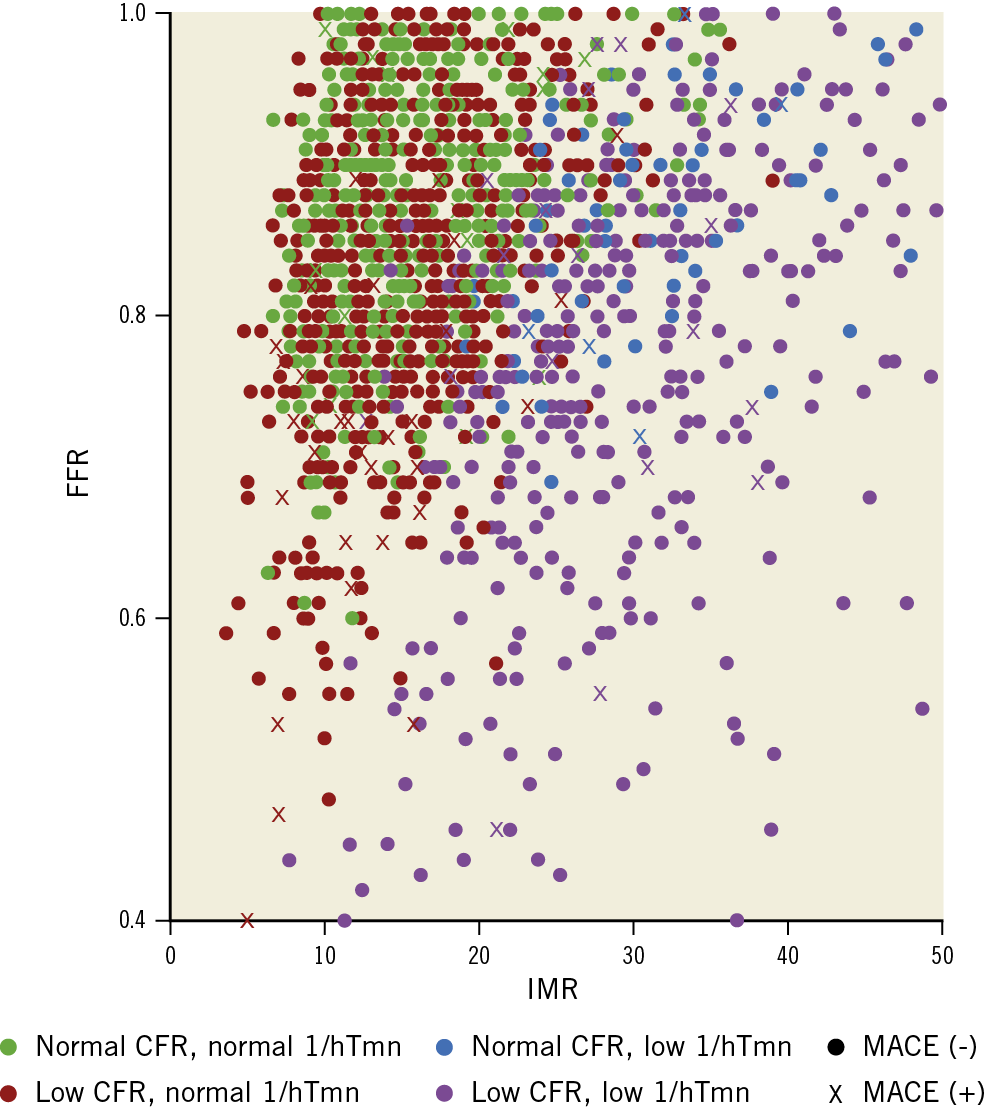
Figure 4. Association of FFR, IMR, CFC, and event. Scatter plots of vessels showing index of microvascular resistance (IMR) and fractional flow reserve (FFR). Colour indicates CFC status: green as CFR >3.2 and 1/hTmn >2.8, red as CFR ≤3.2 and 1/hTmn >2.8, blue as CFR >3.2 and 1/hTmn ≤2.8, and purple as CFR ≤3.2 and 1/hTmn ≤2.8 (low CFC). Crosses indicate vessels that suffered from MACE.
Discussion
This is the first study from a multicentre registry showing different effects of FFR-guided PCI on vessel-related clinical outcomes according to CFC status in stable lesions. FFR-guided PCI might be associated with reduced future adverse events in vessels with low CFC but not in lesions with normal CFC.
CFC AS A POSSIBLE INDICATOR OF FLOW-LIMITING STATUS
The concept of CFC is founded on the rationale that the combination of CFR with hyperaemic flow comprehensively captures all relevant coronary flow characteristics of both epicardial vessels and microvasculature6. Accordingly in the present study, low CFC status could clearly capture epicardial flow disturbance, microvascular dysfunction, or both, which could not be represented solely by either CFR or 1/hTmn. This unique property of CFC suggests that the flow-limiting status of a coronary artery may be distinguished by CFC status, and that the intervention on vessels with low CFC should restore adequate coronary flow. The primary aim of revascularisation in terms of physiology lies in improving coronary blood flow, but it is not always achieved by PCI in stable lesions10,12,13. This may be because the prognostic benefit of PCI in such lesions is determined by balancing the positive aspect of coronary flow restoration and the negative consequence of PCI, such as increased risk of bleeding with antithrombotic therapy, restenosis and need for repeat revascularisation, and stent thrombosis4. We demonstrated a significant interaction between FFR-guided PCI and CFC status for vessel-related future events, suggesting that the balance would be towards favouring the benefit of PCI when indicated for low CFC lesions, but towards emphasising the negative consequences of PCI for normal CFC lesions. We did not observe significant interactions with PCI and CFR or microvascular condition represented as IMR in this cohort (Supplementary Table 1, Supplementary Table 4); therefore, the presence of CFC-defined flow limitation, not microvascular dysfunction, might be important for guiding PCI more effectively in addition to FFR. This needs further verification.
DEFINITION OF CFC
The present thresholds of CFR and hyperaemic flow for defining CFC did not correspond well to those of previous studies. The concept of CFC was first proposed in a PET study using CFR cut-offs representing “ischaemia”. CFC was then examined in intracoronary Doppler-flow technique and thermodilution methods based on the cut-offs corresponding to the PET study7. However, the definition of “ischaemia” has not yet been clearly established with respect to invasively measured regional physiological indices; for example, low regional CFR or FFR is a good representative but does not directly mean ischaemia. Therefore, we explored the definition of CFC statistically in order to highlight the clinical implication, meaning better guidance of PCI in stable lesions. Although this registry was relatively large, the generalisability of the present definition has to be confirmed in prospective trials.
CLINICAL IMPLICATION AND FUTURE DIRECTIONS
Although the present study demonstrated the clinical implication of CFC in guiding elective PCI, the indication should be based primarily on FFR. This is because CFC cannot distinguish the cause of flow limitation (epicardial or microvascular or both) and thus cannot be a single indicator for guidance of elective PCI, a treatment only for epicardial disease. Importantly, the use of CFC with FFR leads to better selection of elective PCI, and CFC can be assessed simultaneously with FFR measurement with little effort using the PressureWire. Based on this hypothesis-generating study, a large-scale, prospective study is warranted to examine whether FFR- and CFC-guided PCI could provide more efficient patient selection for revascularisation compared to FFR-only-guided PCI.
Limitations
This study was conducted based on a global registry consisting of observational studies and cannot escape selection bias. Only approximately 70% of the participants used a statin, which might limit the generalisability considering current practice. The exclusion criteria included left ventricular dysfunction, but a clear, standardised threshold was not leveraged across the institutions of this registry. Insights on the anatomical nature of coronary flow properties such as diffuse disease and tandem lesions were not incorporated. The inter- and intra-observer difference of physiological indices could not be verified because the assessments were performed by many interventionists at multiple international centres. However, the interventionists were instructed to follow standardised protocols; the variability should be minimum. The events were not well adjudicated since the registry was based on three different prospective cohorts. Thermodilution-derived CFR may not serve as the direct reference of PET-defined CFR14. However, that is one of the important reasons why we explored various thresholds of CFR to determine clinically meaningful CFC. The present cut-offs for defining CFC were optimal only in this registry; the generalisability was not evaluated. We could only argue about the concept of CFC for guiding PCI from this study, not the practical definition of CFC, which needs to be assessed in another large registry. The composite outcome was not optimal, as excluding target vessel revascularisation (TVR) from the endpoint significantly reduced the power to detect statistical interaction, while the direction of the effects was similar to the original result (Supplementary Figure 2, Supplementary Table 5). Finally, the present analysis could not identify a statistically significant harmful effect of PCI for lesions with normal CFC at the alpha=0.05 level in a multivariable adjusted model, although the opposite direction of the effect may be plausible. We did demonstrate a significant interaction between PCI and CFC, and the clinical implication was clear.
Conclusions
The effects of FFR-guided PCI on future adverse events were significantly different according to CFC status in stable lesions. PCI to lesions with low CFC was associated with a lower incidence of MACE, while PCI to lesions with normal CFC resulted in increased events, despite the fact that PCI was indicated in these lesions based on low FFR. The integration of CFC with FFR could reduce the number of unnecessary FFR-guided PCIs and optimise the indication of PCI for stable lesions.
|
Impact on daily practice The effect of FFR-guided PCI on vessel-oriented outcomes was significantly different according to the CFC status in stable lesions. FFR-guided PCI for low CFC lesions was associated with reduced incidence of future events, while PCI for normal CFC lesions was not. FFR and CFC could provide complementary information regarding flow limitation. If both indices were used for determining PCI indication, the number of PCIs would decrease by 64%. Our results suggest the potential use of CFC with FFR for optimising the indication for PCI by reducing unnecessary FFR-only-guided PCI. A large-scale, prospective clinical trial is warranted to determine the prognostic impact of CFC guidance when applied complementary to FFR for revascularisation decision making. |
Appendix. Study collaborators
Yoshihisa Kanaji, MD; Yoshinori Kanno, MD; Masahiro Hada, MD; Haruhito Yuki, MD; Masao Yamaguchi, MD; Hiroaki Ohya, MD; Yohei Sumino, MD; Hidenori Hirano, MD; Tomoki Horie, MD; Eisuke Usui, MD; Akinori Sugano, MD, PhD; Tomoyo Sugiyama, MD, PhD; Tadashi Murai, MD, PhD; Division of Cardiovascular Medicine, Tsuchiura Kyodo General Hospital, Ibaraki, Japan. Tetsumin Lee, MD; Department of Cardiovascular Medicine, Tokyo Medical and Dental University, Tokyo, Japan. Ki Hong Choi, MD; Young Bin Song, MD, PhD; Joo-Yong Hahn, MD, PhD; Division of Cardiology, Department of Internal Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea. Doyeon Hwang, MD; Department of Internal Medicine and Cardiovascular Center, Seoul National University Hospital, Seoul, South Korea. Jonghanne Park, MD, PhD; Department of Internal Medicine and Cardiovascular Center, Seoul National University Hospital, Seoul, and Department of Internal Medicine, Naju National Hospital, Ministry of Health and Welfare, Naju, South Korea. Ji-Hyun Jung, MD; Sejong General Hospital, Sejong Heart Institute, Bucheon, South Korea. Hyung Yoon Kim, MD; Department of Cardiovascular Medicine, Chonnam National University Hospital, Gwangju, South Korea. Hae Won Jung, MD; Department of Cardiology, Daegu Catholic University Medical Center, Daegu, South Korea. Yun-Kyeong Cho, MD, PhD; Hyuck-Jun Yoon, MD, PhD; Chang-Wook Nam, MD, PhD; Seung-Ho Hur, MD, PhD; Department of Medicine, Keimyung University Dongsan Medical Center, Daegu, South Korea. Joon-Hyung Doh, MD, PhD; Department of Medicine, Inje University Ilsan Paik Hospital, Goyang, South Korea. Eun-Seok Shin, MD, PhD; Division of Cardiology, Ulsan Hospital, Ulsan, Korea and Department of Cardiology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, South Korea. Hernán Mejía-Rentería, MD; Francesco Lauri, MD; Sonoka Goto, MD; Fernando Macaya, MD; Angela McInerney, MD; Giacomo Gravina, MD; Rafael Vera, MD; Nieves Gonzalo, MD, PhD; Pilar Jimenez-Quevedo, MD, PhD; Ivan Nuñez-Gil, MD; Pablo Salinas, MD, PhD; Luis Nombela-Franco, MD, PhD; Maria del Trigo, MD; Antonio Fernández-Ortiz, MD, PhD; Carlos Macaya, MD, PhD; Cardiovascular Institute, Hospital Clinico San Carlos, Madrid, Spain.
Conflict of interest statement
B.-K. Koo has received an institutional research grant from St. Jude Medical (Abbott Vascular) and Philips Volcano. I. Nuñez Gil has received personal fees from AstraZeneca/Medtronic outside the submitted work. J.-Y. Hahn has received a research grant from St. Jude Medical (Abbott Vascular). P. Salinas has received speaker fees from Terumo, Alvimedica, Biomenco, and Boston Scientific. J. Escaned has received personal fees from Philips Volcano, Boston Scientific, and Abbott/St. Jude Medical, outside the submitted work. All other authors/collaborators declare that there is no conflict of interest relevant to the submitted work.
Supplementary data
To read the full content of this article, please download the PDF.

