
Abstract
Aims: The aim of this study was to investigate the prognostic value of thermodilution-derived coronary flow capacity (T-CFC) in patients with stable coronary artery disease and deferred revascularisation.
Methods and results: We evaluated 308 lesions in 308 patients with deferred revascularisation, stratifying the cohort according to T-CFC. Ischaemic T-CFC was defined as a composite of mildly, moderately, and severely reduced T-CFC. Clinical outcomes were assessed by vessel-oriented composite endpoints (VOCE) and major adverse cardiac events (MACE). VOCE and MACE occurred in 19 and 28 patients, respectively. Ischaemic T-CFC was found in 88 lesions (28.6%). Kaplan-Meier analysis revealed that lesions with ischaemic T-CFC had a significantly higher risk of both VOCE and MACE. The net reclassification index and integrated discrimination improvement index were both significantly improved when ischaemic T-CFC was added to the clinical risk model (age, sex, prior stent implantation, and lesion length) for predicting VOCE and MACE. Furthermore, ischaemic T-CFC showed significant incremental predictive ability for VOCE and MACE when compared with the clinical risk model + fractional flow reserve ≤0.8, or with the clinical model + coronary flow reserve ≤2.0.
Conclusions: T-CFC categorisation improved the risk stratification for both VOCE and MACE and showed incremental prognostic value in patients with deferred revascularisation.
Introduction
Fractional flow reserve (FFR)-guided revascularisation is recommended in updated clinical guidelines1. The benefits of physio-logy-guided decision making in revascularisation are largely attributable to deferral of percutaneous coronary intervention (PCI)2,3. A recent meta-analysis by Zimmermann et al showed that FFR-guided PCI resulted in a reduction of the composite of cardiac death or myocardial infarction (MI) compared with medical therapy, which was driven by a decreased risk of MI4. For deferred lesions in one prospective large registry study, the risk of major adverse cardiac events (MACE) demonstrated a significant, inverse relationship with FFR when lesions were deferred after FFR measurements5. However, although FFR is highly reli-able for physiological assessment of epicardial lesions, the severity and extent of microvascular dysfunction that occurs irrespective of epicardial stenosis could not be determined by FFR. A comprehensive diagnostic approach to coronary heart disease is warranted based on prior evidence indicating a strong link between adverse clinical outcomes and microcirculatory disturbance as well as epicardial flow impairment6.
Coronary flow reserve (CFR), another index of coronary flow assessment, indicates integrated coronary vascular function, which is known to be associated with adverse cardiac events7,8. Several previous studies have evaluated the prognostic value of CFR, and CFR was consistently reported to be associated with clinical outcomes9,10. The problems when using CFR relate to unstable baseline haemodynamics, attenuated hyperaemic responses caused by various conditions, and a heterogeneous population with low CFR including patients with high coronary flow at baseline, limited flow with hyperaemia, or impaired vasodilatory response to adenosine. To overcome the limitations of CFR, coronary flow capacity (CFC) was introduced, which integrates CFR with hyperaemia coronary flow into a comprehensive platform of coronary flow characteristics11. CFC was first developed using non-invasive positron emission tomography (PET), subsequently verified using an invasive intracoronary Doppler flow wire12, and recently evaluated using a pressure-temperature sensor-tipped wire13. Despite the strong theoretical fundamentals of CFC, validation of CFC for potential clinical use is needed to determine whether the prognostic implications are better than for CFR alone. In the present study, we investigated the prognostic efficacy of CFC obtained using a pressure-temperature sensor-tipped wire (thermodilution method [T-CFC]) in patients with deferred revascularisation based on FFR and clinical judgement. Of note, FFR can be determined with CFR and T-CFC categorisation using the same wire. Thus, we hypothesised that T-CFC could provide incremental prognostic information for subsequent adverse events compared with FFR or CFR alone.
Methods
PATIENT POPULATION
From June 2012 to June 2017, patients with known or suspected coronary artery disease who underwent coronary physiological assessments using the PressureWire™ (St. Jude Medical, St. Paul, MN, USA) at Tsuchiura Kyodo General Hospital were identified from the institutional physiology database. We enrolled patients with deferred revascularisation after physiological examination of intermediate lesions documented in the institutional physiology data registry. A physiological study was indicated for vessels with intermediate coronary lesions (30%-80% diameter stenosis on visual assessment). With multiple coronary stenoses, we used a single vessel with the most severely decreased FFR value. The exclusion criteria are detailed in Supplementary Appendix 1.
This study was conducted in accordance with the Declaration of Helsinki; our institutional ethics committee approved the study protocol. Before catheterisation, all patients provided written informed consent for enrolment in the institutional database for potential future investigations. All patient data and procedural details were obtained from medical records, and prompt optimal medical therapy was initiated in all patients after coronary angiography (CAG).
CORONARY PHYSIOLOGICAL ASSESSMENT
FFR, mean transit time (Tmn), and index of microvascular resistance (IMR) were determined using a RadiAnalyzer™ Xpress instrument with a PressureWire™ Certus™ (St. Jude Medical) as previously described14,15. Details are shown in Supplementary Appendix 1.
DERIVATION OF CORONARY FLOW CAPACITY
Based on CFC values derived from PET or Doppler flow velocity, T-CFC categorises lesions into four ranges using CFR and the inverse of hyperaemic Tmn11,12. The inverse of hyperaemic Tmn (1/Tmn) can be a surrogate that correlates well with absolute hyperaemic coronary flow because shorter Tmn suggests higher coronary flow velocity15. Because thresholds or cut-off values for 1/Tmn have not been well established, we matched Tmn values according to the percentiles corresponding to CFR values as follows: normal T-CFC indicated no myocardial ischaemia and CFR ≥2.80 with corresponding 1/Tmn >3.70 (53th percentile)16, mildly reduced T-CFC indicated CFR <2.80 and ≥2.20, which were the reported upper limits for inducible ischaemia, and a corresponding 1/Tmn <3.70 and ≥2.7 (72nd percentile each)17, moderately reduced T-CFC indicated CFR <2.20 and ≥1.90, which reflected lower limits for inducible ischaemia, and 1/Tmn <2.70 and ≥2.30 (80th percentile each)18, and severely reduced T-CFC indicated definite ischaemia with CFR <1.90 and 1/Tmn <2.3019. We defined ischaemic T-CFC as a composite of mildly, moderately, and severely reduced T-CFC.
CLINICAL FOLLOW-UP
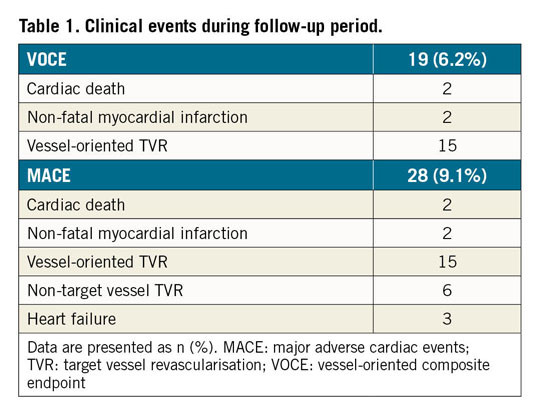
The primary outcome was the vessel-oriented composite endpoint (VOCE), including cardiovascular death, vessel-related spontaneous MI, and ischaemia-driven target vessel revascularisation (TVR). The secondary endpoint was MACE, including VOCE, non-TVR, and heart failure requiring hospitalisation.
STATISTICAL ANALYSIS
Statistical analyses were performed using SPSS, Version 23.0 (IBM Corp., Armonk, NY, USA). Categorical data were expressed as numbers and percentages and compared by χ2 or Fisher’s exact tests, as appropriate. Continuous biochemical or physiological data were expressed as median (interquartile range [IQR]) and analysed using the Mann-Whitney test and analysis of variance for variables with non-normal distribution and normal distribution, respectively. Correlations between the two parameters were evaluated using linear regression analysis. Receiver operating characteristic curves were analysed to assess the best cut-off values for the physiological indices and clinical characteristics to predict the occurrence of VOCE and MACE; the optimal cut-off was calculated using Youden’s index (Supplementary Figure 1). Event rates over time were estimated using the Kaplan-Meier method, and linear trends were tested with log-rank tests. A Cox proportional hazards regression model was used to identify independent predictors of VOCE and MACE, and the covariates used in multivariate analysis were selected using the criterion of p<0.10 in the univariate analysis. A collinearity index was used to evaluate linear combinations among covariates with the Akaike information criterion to avoid overfitting. Ten prediction models were constructed to determine the incremental discriminatory and reclassification performance of physiological parameters for VOCE or MACE by using relative integrated discrimination improvement (IDI) and category-free net reclassification index (NRI). Prediction models are detailed in Supplementary Appendix 1; p<0.05 indicated statistical significance.
Results
BASELINE PATIENT CHARACTERISTICS AND PROCEDURAL FINDINGS
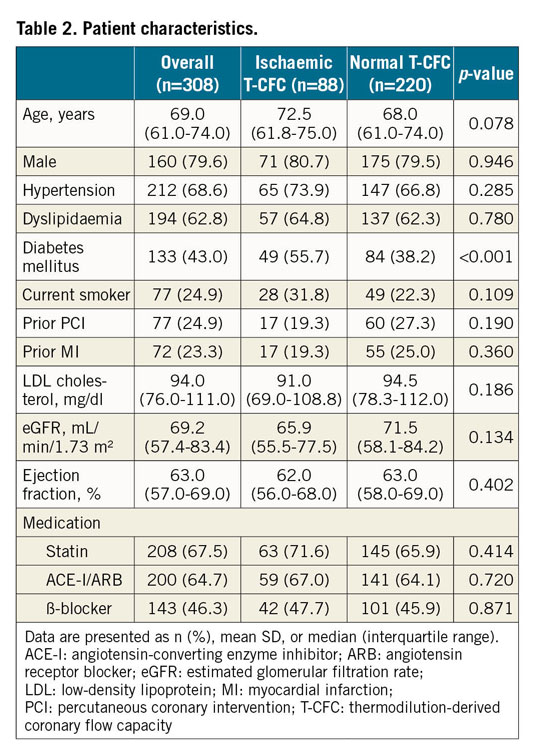
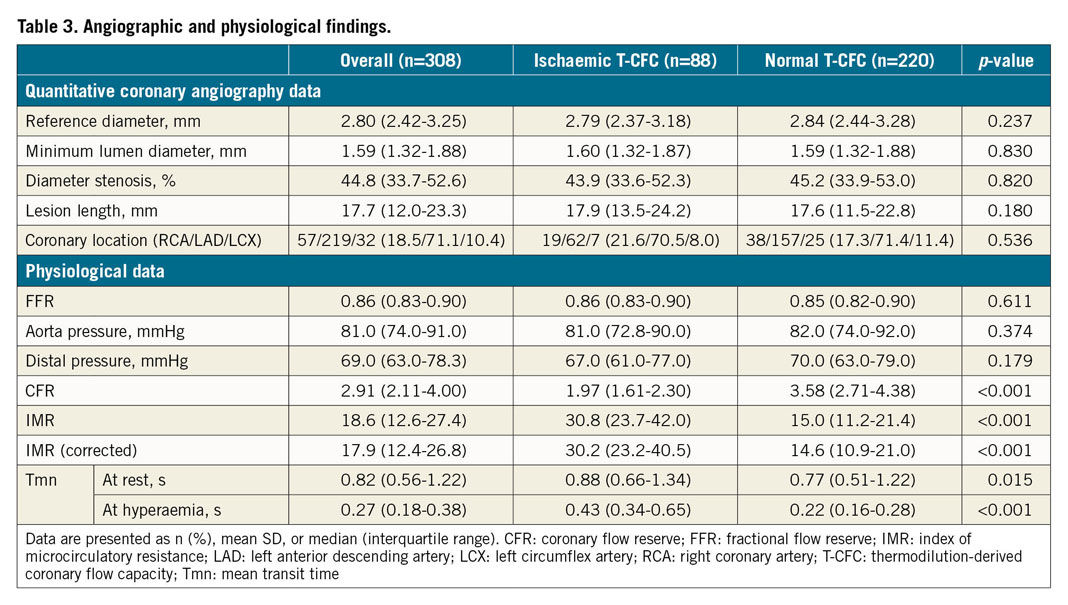
A total of 308 patients with 308 lesions were included in the present analysis (Figure 1). Median FFR and CFR values were 0.86 (IQR: 0.83–0.90) and 2.91 (IQR: 2.11-4.00), respectively. We created the T-CFC map using CFR and the inverse of hyperaemic Tmn, and categorised patients into four T-CFC categories (Figure 2). Normal, mildly, moderately, and severely reduced T-CFC categories constituted 220 (71.2%), 48 (15.5%), 18 (5.8%), and 22 (7.1%) vessels, respectively. Next, we defined the composite of mildly, moderately, and severely reduced T-CFC as ischaemic T-CFC (n=88, 28.5%). Clinical outcomes are summarised in Table 1. The baseline clinical characteristic and physiological parameters for each T-CFC group are summarised in Table 2. Compared with normal T-CFC, the prevalence of patients with diabetes mellitus was significantly greater in ischaemic T-CFC. The physiological properties of the lesions differed significantly between the normal T-CFC and ischaemic T-CFC groups, although a non-significant difference in FFR was seen (Table 3). Ischaemic T-CFC vessels were significantly associated with microvascular dysfunction.
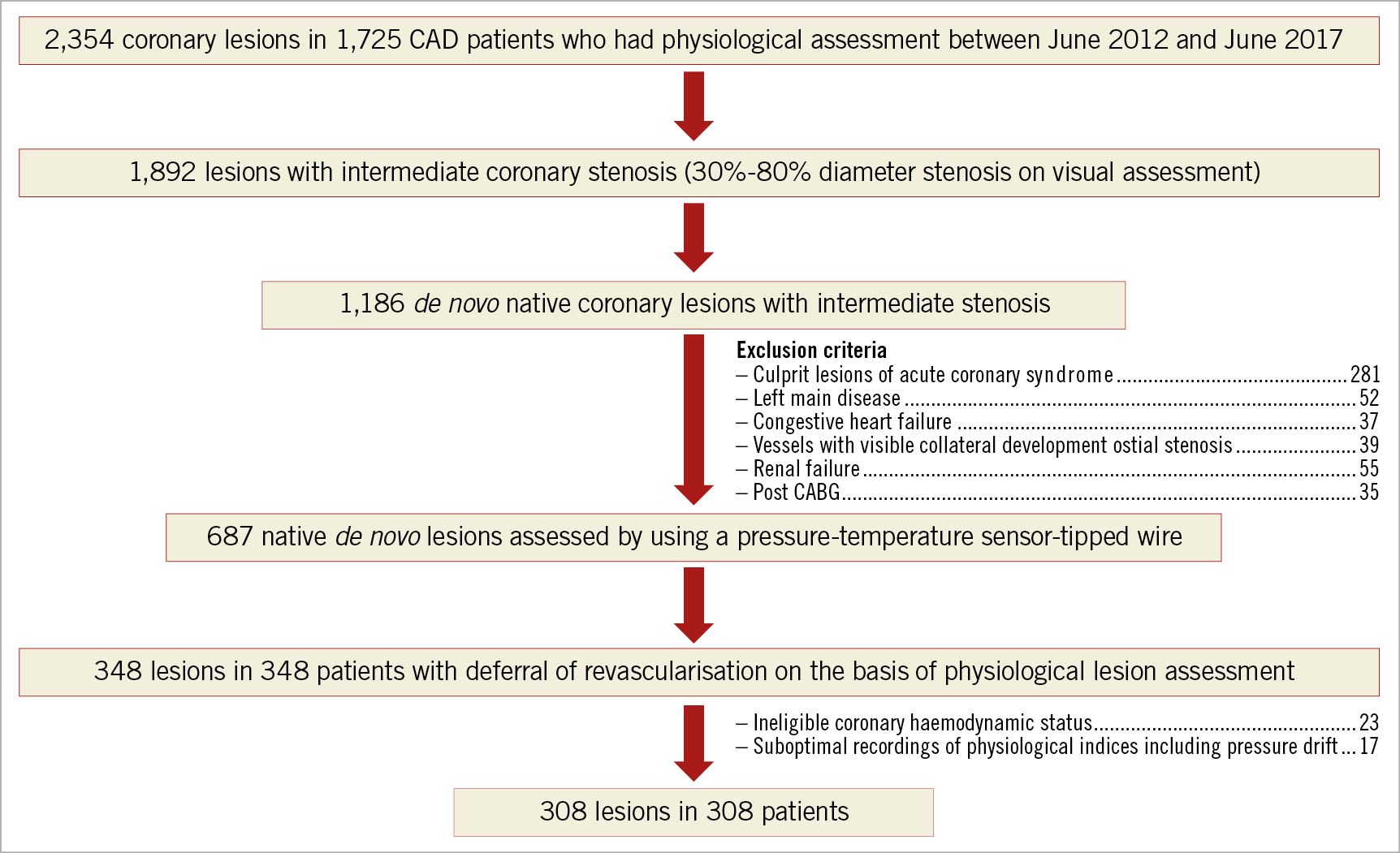
Figure 1. Study population. CABG: coronary artery bypass graft; CAD: coronary artery disease
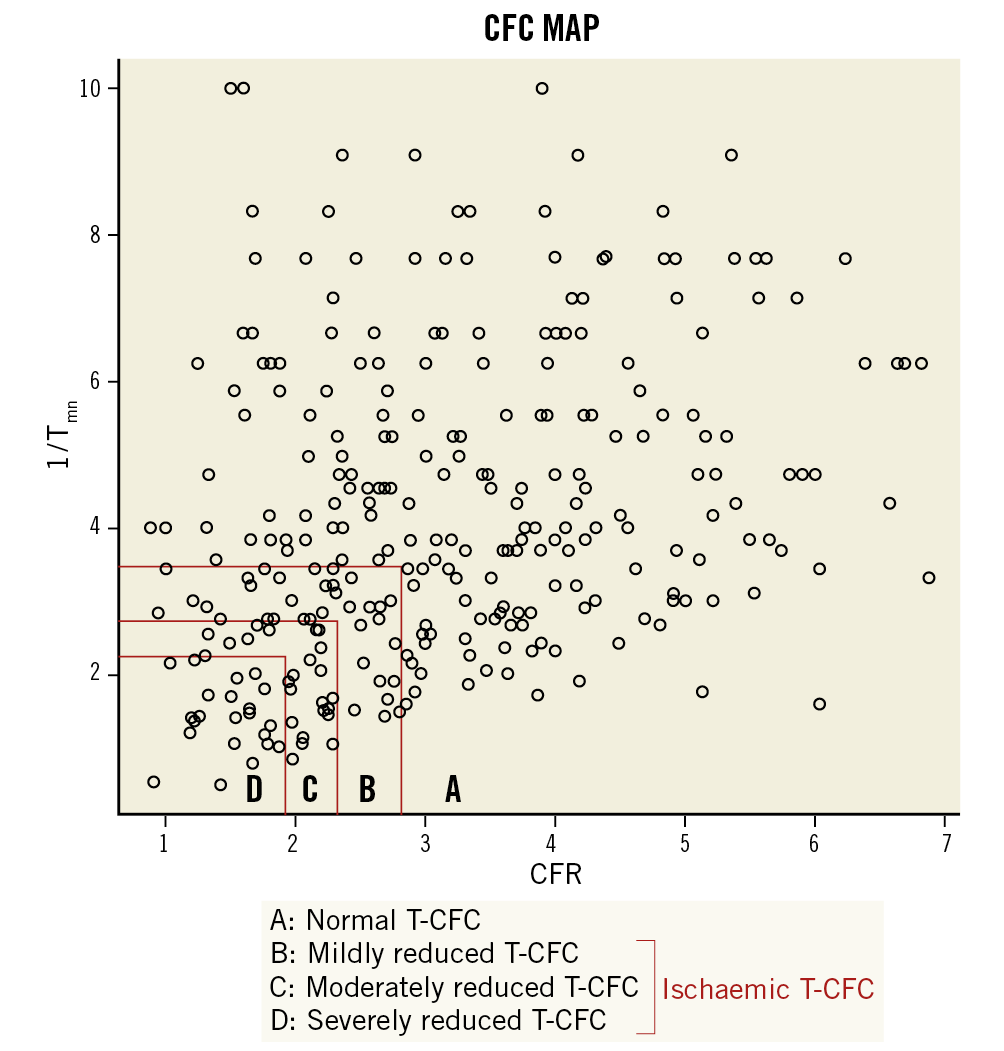
Figure 2. Distribution of 308 lesions across the two-dimensional map of CFR versus 1/Tmn values with four categories. A) A total of 220 lesions (71.2%) showed normal T-CFC. B) 45 (15.5%) showed mildly reduced T-CFC. C) 18 (5.8%) showed moderately reduced T-CFC. D) 22 (7.1%) showed severely reduced T-CFC. CFR: coronary flow reserve; T-CFC: thermodilution-derived coronary flow capacity; Tmn: mean transit time
CLINICAL OUTCOMES
During the median follow-up of 30 months (range, 20-61 months), VOCE occurred in 19 (6.2%) patients and MACE occurred in 28 (9.1%) patients. Demographics, angiographic and procedural characteristics according to the presence or absence of VOCE and MACE are shown in Supplementary Table 1. There were no significant differences in baseline characteristics in the two patient groups regarding the presence or absence of VOCE (19 events) or MACE (28 events). Lesions with FFR ≤0.8 predicted both VOCE and MACE, while CFR ≤2.0 predicted MACE, specifically (Figure 3, Figure 4). Kaplan-Meier analysis revealed that lesions with ischaemic T-CFC had a significantly higher risk of both VOCE and MACE (Figure 5). Kaplan-Meier curves showing survival from MACE according to all four T-CFC categories appear in Supplementary Figure 2. Multivariate Cox proportional hazards analysis demonstrated that ischaemic T-CFC and FFR ≤0.8 were independent predictors of VOCE (Supplementary Table 2). Multivariate Cox proportional hazards analysis demonstrated that age, prior stent implantation, and ischaemic T-CFC were independent predictors of MACE (Supplementary Table 3). IMR was not a significant factor for predicting VOCE or MACE. NRI and IDI index were both significantly improved when ischaemic T-CFC was added to the clinical risk model 1 for predicting VOCE and MACE. Furthermore, ischaemic T-CFC showed significant incremental predictive ability when compared with the clinical risk model + FFR ≤0.8 or the clinical model + CFR ≤2.0 (Figure 6). In the subgroup analysis of deferred patients with FFR >0.8, ischaemic CFC was also significantly associated with poor prognosis (Supplementary Figure 3), whereas CFR ≤2.0 showed no significant predictive information (Supplementary Figure 4). Furthermore, ischaemic T-CFC showed significant incremental predictive ability when compared with the clinical risk model + CFR ≤2.0 (Supplementary Figure 5).
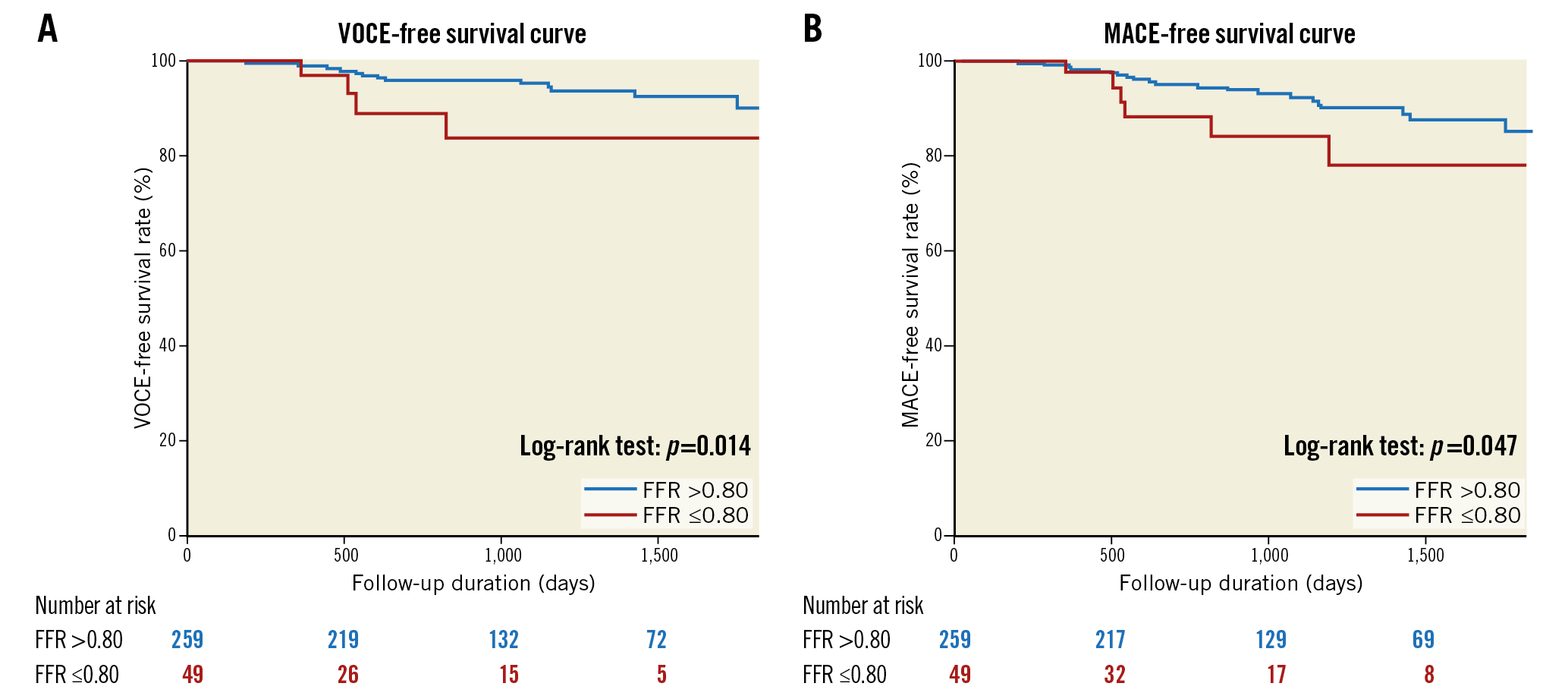
Figure 3. Survival from cardiac events in patients based on an FFR value of 0.8. A) Kaplan-Meier curves demonstrating survival from VOCE in patients based on an FFR value of 0.8. B) Kaplan-Meier curves demonstrating survival from MACE in patients based on an FFR value of 0.8. FFR: fractional flow reserve; MACE: major adverse cardiac events; VOCE: vessel-oriented composite endpoint
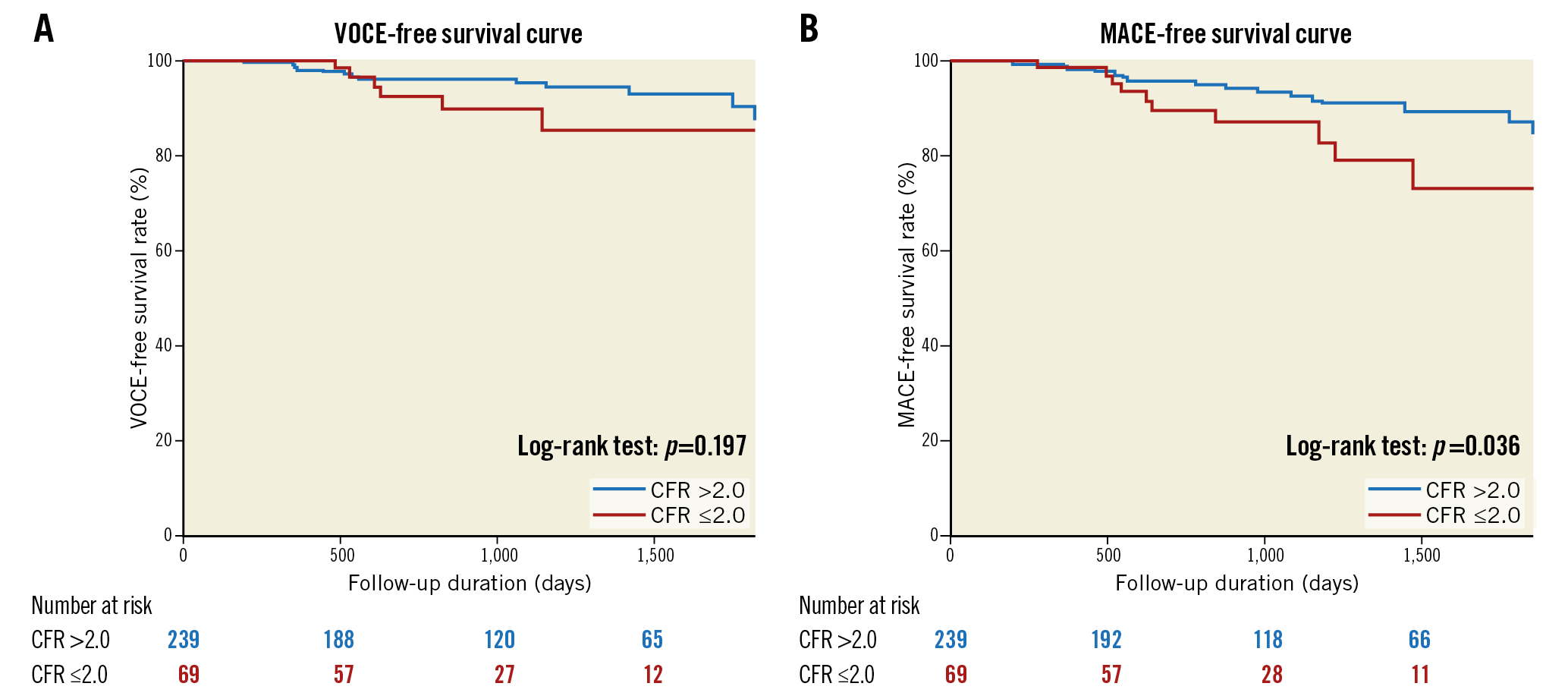
Figure 4. Survival from cardiac events in patients based on a CFR value of 2.0. A) Kaplan-Meier curves demonstrating survival from VOCE in patients based on a CFR value of 2.0. B) Kaplan-Meier curves demonstrating survival from MACE in patients based on a CFR value of 2.0. CFR: coronary flow reserve; MACE: major adverse cardiac events; VOCE: vessel-oriented composite endpoint
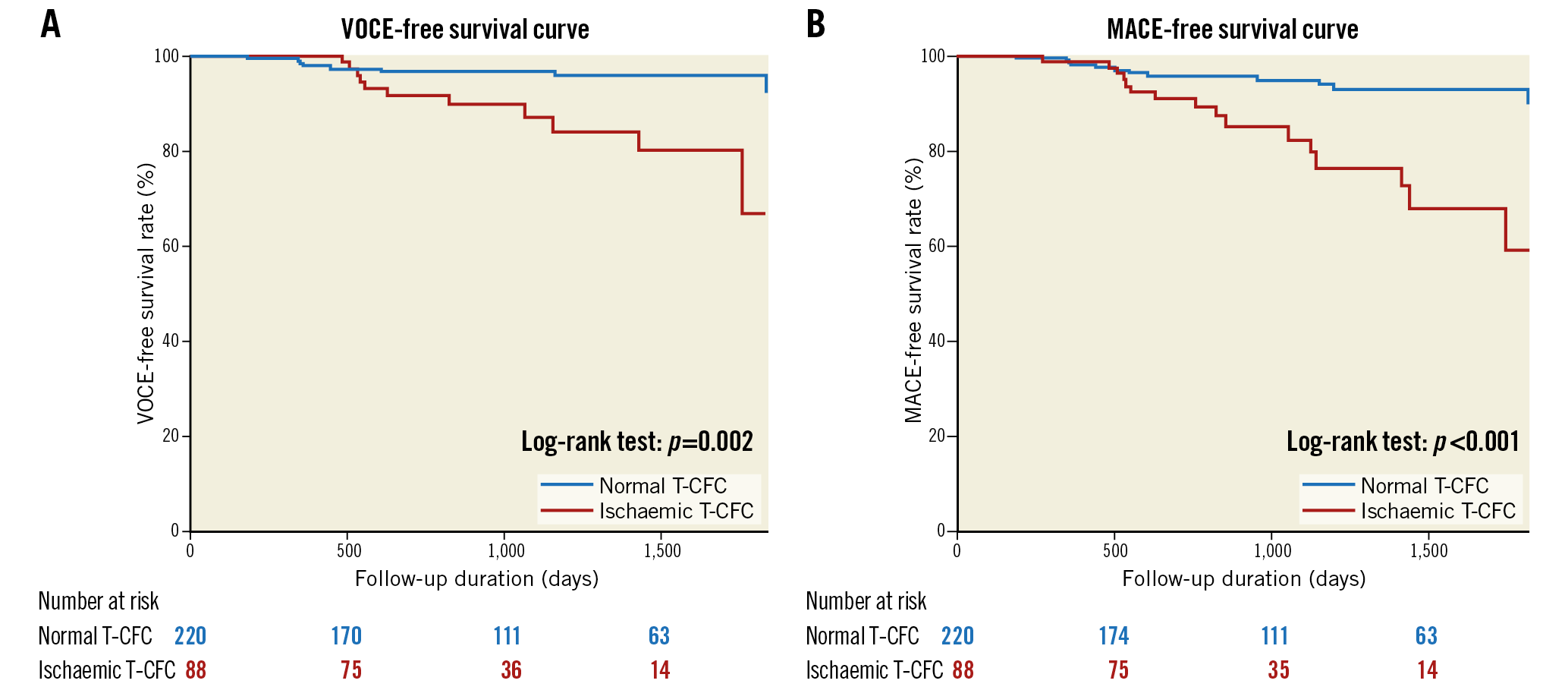
Figure 5. Survival from cardiac events in patients divided into two groups by T-CFC. A) Kaplan-Meier curves demonstrating survival from VOCE in patients divided into two groups by T-CFC. B) Kaplan-Meier curves demonstrating survival from MACE in patients divided into two groups by T-CFC. MACE: major adverse cardiac events; T-CFC: thermodilution-derived coronary flow capacity; VOCE: vessel-oriented composite endpoint
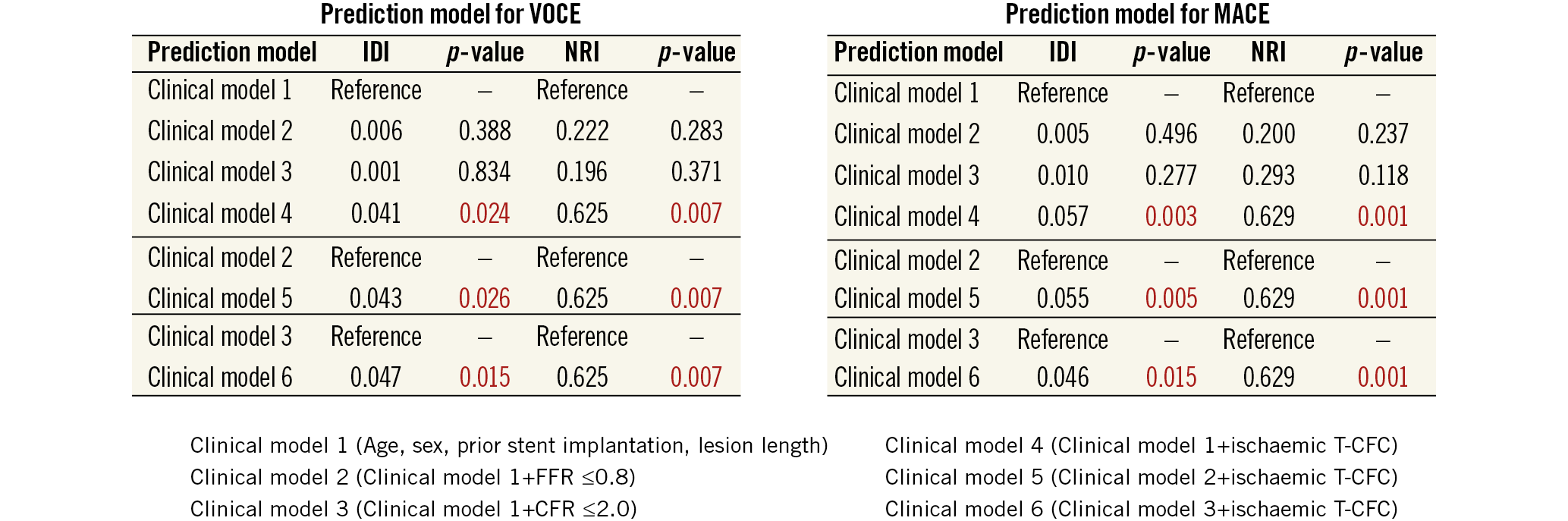
Figure 6. Comparison of discriminant and reclassification ability of predictive models to determine incremental discriminatory and reclassification capacities of FFR, CFR and T-CFC for cardiac events. Red numbers indicate statistical significance. CFR: coronary flow reserve; FFR: fractional flow reserve; IDI: relative integrated discrimination improvement; MACE: major adverse cardiac events; NRI: net reclassification index; T-CFC: thermodilution-derived coronary flow capacity; VOCE: vessel-oriented composite endpoint
Discussion
To our knowledge, this is the first study demonstrating that categorisation based on coronary flow capacity improved risk stratification and showed incremental prognostic value for both VOCE and MACE compared with FFR or CFR alone in patients with deferred revascularisation for stable coronary lesions. Patients with deferred lesions with ischaemic T-CFC showed a significantly higher risk of VOCE and MACE than patients with lesions with normal T-CFC.
Although FFR has become the standard in decision making for revascularisation, FFR >0.8 does not necessarily mean the absence of ischaemia or that a patient is free from the risk of adverse events20. In the FAME 2 trial, patients with stenoses and FFR >0.8 who were treated by optimal medical therapy alone still suffered MACE in more than 10% of evaluated vessels3. Although FFR measurement is highly feasible and valuable in daily clinical practice, findings suggest that comprehensive assessment of coronary artery disease including diffuse arterial narrowing and microvascular dysfunction, which indicate risk of future clinical adverse outcomes, is needed to predict subsequent adverse events after revascularisation deferral based on FFR21. Johnson and Gould11 first proposed CFC, based on the rationale that combining CFR with hyperaemic flow comprehensively captures all relevant flow characteristics of the evaluated vasculature. Despite the strong theoretical fundamentals of CFC, validation is necessary for potential clinical use to determine whether the prognostic implications are better than with FFR or CFR alone. To date, the clinical usefulness of CFC obtained by pressure-temperature sensor-tipped wire-derived coronary flow capacity in patients with deferred revascularisation has not been evaluated and, to our knowledge, our results are the first to demonstrate the superiority of T-CFC to predict VOCE or MACE compared with FFR or CFR alone in these patients. Worse prognoses in the ischaemic T-CFC group in the present study may be related to the prevalent microvascular dysfunction that is not evaluated by FFR, described as high IMR in patients with ischaemic T-CFC (Table 3). Regarding the underlying mechanism of clinical events despite functionally insignificant epicardial coronary stenosis, previous studies have suggested a link between the presence of microvascular disease, endothelial dysfunction, subclinical inflammation, unexpected rapid atherosclerotic progression, and coronary vasomotor dysfunction with subsequent adverse events22,23,24. Lee et al recently reported that, in patients with functionally non-significant stenoses (FFR >0.8, n=230), clinical outcomes were the worst in those with both elevated IMR and low CFR25. In our study, IMR was not a significant factor for predicting VOCE or MACE, whereas T-CFC did identify high-risk patients for both VOCE and MACE. Of note, T-CFC effectively stratified both CFR and IMR in our population (Table 3), and lesions in the ischaemic T-CFC group showed significantly higher IMR compared with those in the normal T-CFC group, similar to results in the study by Lee et al. Both in the study by Lee et al and in our studies, outcome events were driven mainly by unplanned remote revascularisation in patients with ischaemic T-CFC. These results suggest that microvascular dysfunction and other vascular functional impairment, including diffuse coronary disease and endothelial dysfunction potentially represented by reduced T-CFC, may be a marker of subsequent epicardial lesion progression requiring PCI in deferred patients with lesions showing non-ischaemic FFR values. Our results showing that T-CFC could discriminate patients at high risk of both VOCE and MACE suggest that T-CFC may be a marker of both target lesion information and global atherosclerotic burden susceptible to adverse events or stenosis progression.
The specific aspects and aims of the present study which were different from our previously published paper13 are detailed in Supplementary Appendix 2.
In the original CFC classification based on average peak flow velocity (APV)12, the ischaemic T-CFC is considered as the moderately and severely reduced T-CFC. On the other hand, we defined “ischaemic” T-CFC as the T-CFC from mild to severe impairment. The details of difference between the original one and our definition are in Supplementary Appendix 2. Comparison of Doppler techniques and thermodilution method are also detailed in Supplementary Appendix 2.
Study limitations
Our results should be interpreted bearing in mind several important limitations. First, this study included a relatively small number of patients from a single centre, which may not allow extensive subgroup analysis or more reliable multivariable analyses. Second, rigorous exclusion criteria limited the number of included patients. The small number of events precludes the differentiation of hard endpoints, including death and MI, which may be more important than emergent revascularisation, considering prevention. With no established cut-off values for hyperaemic Tmn, the proposed cut-off values were derived from the percentiles of hyperaemic Tmn corresponding to CFR cut-offs defined by the present population. The use of Tmn had an important intrinsic limitation which showed the wider distribution of T-CFC compared with APV-based CFC. Furthermore, 1/Tmn is a surrogate index of absolute flow, which depends on the size of the perfused myocardial territory, being larger in proximal locations and smaller in distal segments. Theoretically, Doppler flow velocity-derived T-CFC is more accurate, because the decrease in flow velocity from proximal to distal segments is much smaller than the decrease in volumetric flow. Although obtaining high-quality Doppler flow velocity data remains challenging, Doppler flow velocity showed superior agreement of CFR with [15O] H2O PET compared with thermodilution26. The thermodilution-derived CFC concept accompanies these several limitations and might show different features compared with Doppler APV-based CFC or PET-based CFC. Moreover, CFC may not differentiate flow impairment between the epicardial and microcirculatory domains of the coronary circulation.
Conclusions
T-CFC mapping provided accurate predictions of coronary flow impairment. Categorisation by T-CFC was associated with the incidence of VOCE and MACE independently from FFR or CFR in patients with deferred revascularisation lesions. Further studies are needed to validate our hypothesis and results regarding the implications of T-CFC.
|
Impact on daily practice T-CFC categorisation improved the risk stratification for both VOCE and MACE in patients with deferred revascularisation. T-CFC categorisation showed incremental predictive value compared with FFR or CFR alone in patients with deferred revascularisation. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

