The novel results presented in this issue of EuroIntervention from an international collaboration on intracoronary physiologic assessment1 provide a timely opportunity to critically assess the concept of coronary flow capacity (CFC) that we introduced in 20122.
Why coronary flow capacity?
Due to autoregulation under baseline conditions that maintains myocardial blood flow over a wide range of coronary pressures (and hence tolerates a surprising burden of epicardial atherosclerosis), only stress measurements allow a causal link with symptoms or outcomes in stable patients. Yet two separate conceptual metrics exist as candidates: hyperaemic flow (or perfusion, if adjusted for supplied myocardial mass) and coronary flow reserve (CFR), the unitless ratio of hyperaemic to baseline flow. Prior literature has variably found that both peak flow and CFR provide independent prognostic value as well as being associated with adverse surrogates such as reduced fractional flow reserve (FFR) or frank ischaemia during vasodilator stress.
Our introduction of CFC in 20122 sought to avoid an either/or response to the natural question of which parameter is more important. Rather, we proposed that the myocardium cares about both aspects. In other words, we must assess peak flow as well as the ability to increase flow in response to a stress stimulus. By doing so, we can understand two seemingly discordant patterns with a similar prognosis, namely intact stress flow but reduced CFR (due to elevated resting flow as commonly observed in female, hypertensive, and kidney patients) and intact CFR yet reduced stress flow (due to low resting flow as often desired therapeutically in patients with aggressive medical treatment of the pressure-rate product).
What about the latest results?
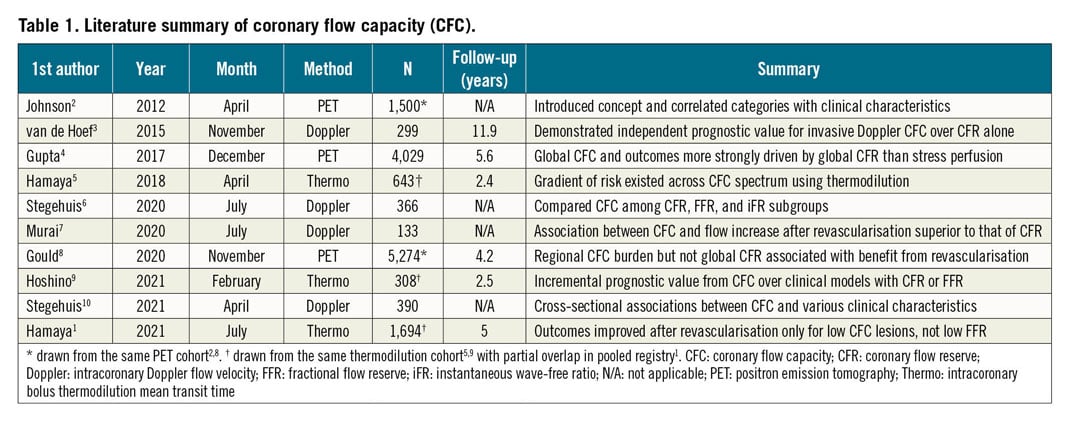
Table 1 summarises, in chronological order, the major publications studying CFC1,2,3,4,5,6,7,8,9,10, evaluated using both non-invasive and invasive tools. The latest result1 adds two new findings to the existing literature. First, it used subsequent clinical outcomes to determine thresholds for CFR and peak flow (in this case 1/hTmn, the inverse of hyperaemic mean transit time during bolus thermodilution). As anticipated mechanistically, a broadly lower threshold was observed for composite cardiovascular death and target vessel infarction (CFR=1.7 and 1/Tmn=3.2) than when including target vessel revascularisation (higher CFR=3.2 and similar 1/Tmn=2.8). Second, a significant interaction existed between clinical outcomes after percutaneous coronary intervention (PCI) and binary CFC status. Intriguingly, only vessels with reduced CFC showed a reduction in long-term events after PCI.
Where to next?
As with any new diagnostic test, it only makes sense to study its disagreements with existing clinical pathways. Invasively, FFR has rightly become the reference standard for choosing between revascularisation and medical therapy. Can vessels with an abnormal FFR yet intact CFC be treated medically without a significant increase in angina or spontaneous infarction? Alternatively, should we revascularise vessels with abnormal CFC despite intact FFR to reduce those same endpoints? Non-invasively, perfusion imaging classically focuses on stress-induced relative uptake defects. Should we refer patients with low CFC for cardiac catheterisation even in the absence of a significant relative perfusion defect? And can we conservatively treat patients whose stress-induced defect has intact CFC?
The past decade has witnessed the introduction of CFC and its retrospective application to almost 10,000 PET scans and 3,000 vessels with average follow-up between 2.5 and 12 years1,2,3,4,5,6,7,8,9,10. In other words, CFC has already been independently studied globally in thousands of patients over the long term. We look forward to the next decade of CFC, whose goal should be its prospective application, ideally in randomised outcomes trials. Finally, the fundamental relations of all epicardial artery pressure/flow measurements, stress perfusion, CFR, and CFC to transmural perfusion gradients, subendocardial perfusion, or ischaemia remain to be defined and quantified.
Conflict of interest statement
N.P. Johnson and K.L. Gould have received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis, and have patents pending on diagnostic methods for quantifying aortic stenosis and TAVI physiology, and on methods to correct pressure tracings from fluid-filled catheters. N.P. Johnson has received significant institutional research support from St. Jude Medical (CONTRAST, NCT02184117) and Philips/Volcano (DEFINE-FLOW, NCT02328820) for studies using intracoronary pressure and flow sensors, and has an institutional licensing agreement with Boston Scientific for the smart minimum FFR algorithm commercialised under 510(k) K191008. K.L. Gould is the 510(k) applicant for CFR Quant (K113754) and HeartSee (K143664 and K171303, K202679), software packages for cardiac positron emission tomography image processing, analy-sis, and absolute flow quantification.
Supplementary data
To read the full content of this article, please download the PDF.

