
Abstract
Aims: There are limited data on the prognosis of deferred non-culprit lesions in patients with acute coronary syndrome (ACS) based on fractional flow reserve (FFR). We aimed to investigate the prognosis of deferred non-culprit lesions in ACS patients, compared with deferred lesions in patients with stable coronary artery disease (SCAD), on the basis of FFR.
Methods and results: The clinical outcomes of 449 non-culprit lesions (301 patients with ACS) were compared with 2,484 lesions (1,295 patients with SCAD) in which revascularisation was deferred on the basis of a high FFR (>0.80). The primary outcome was major adverse cardiac events (MACE), a composite of cardiac death, target vessel-related myocardial infarction (MI) and ischaemia-driven revascularisation. Among the ACS population, 65.8% presented with unstable angina and 34.2% with non-ST-segment elevation MI. Mean angiographic percent diameter stenosis and FFR of the deferred lesions were 39.3±15.0% and 0.92±0.06, respectively. During the median follow-up duration of 722.0 days, the deferred non-culprit lesions of ACS patients showed a significantly higher rate of MACE (3.8% vs. 1.6%, HRadj 2.97, 95% CI: 1.23-7.17, p=0.016), mainly driven by the higher rate of ischaemia-driven revascularisation (2.8% vs. 1.1%, HRadj 3.39, 95% CI: 1.29-8.92, p=0.013) than the deferred lesions in SCAD patients. Regardless of the range of FFR in the deferred lesions (0.81-0.85, 0.86-0.90, 0.91-0.95, and 0.95-1.00), non-culprit lesions of ACS showed a more than twofold higher rate of MACE than that of SCAD. In a multivariable marginal Cox model, ACS was the most powerful independent predictor of MACE (HRadj 2.74, 95% CI: 1.13-6.64, p=0.026).
Conclusions: Compared to the deferred lesions of SCAD patients, deferred non-culprit lesions of ACS on the basis of FFR showed a higher rate of clinical events, regardless of FFR range.
Abbreviations
ACS: acute coronary syndrome
CABG: coronary artery bypass graft surgery
CI: confidence interval(s)
FFR: fractional flow reserve
HRadj: adjusted hazard ratio
MACE: major adverse cardiac events
MI: myocardial infarction
NSTEMI: non-ST-segment elevation myocardial infarction
SCAD: stable coronary artery disease
STEMI: ST-elevation myocardial infarction
Introduction
Fractional flow reserve (FFR)-guided decision making has been regarded as a standard invasive method to evaluate the functional significance of epicardial coronary artery stenosis. The clinical outcomes of an FFR-guided percutaneous coronary intervention (PCI) strategy were reported to be better than those of angiography-guided PCI or medical treatment1. This study on the FFR-guided strategy was performed mainly in patients with stable coronary artery disease (SCAD).
In patients with acute coronary syndrome (ACS), individual variation and the unpredictable nature of microvascular function in the culprit vessels may preclude FFR for proper assessment of the functional significance of coronary stenosis2. Although previous studies have shown the benefit of patient-level FFR-guided decision making in patients with ACS and multivessel disease3,4, there have been debates regarding the clinical relevance of per-lesion decisions based on FFR, especially for the culprit lesion5-9. A recent study by Hakeem et al showed significantly worse clinical outcomes of deferred culprit lesions according to FFR compared with those of SCAD8 and proposed a cut-off value of FFR <0.84 in the culprit vessel of ACS patients.
Rather than the evaluation of culprit lesions, more common situations for FFR use in real-world practice would be the evaluation of non-culprit lesions in ACS patients. Although the reliability of FFR measurement for non-culprit lesions in ACS patients was reported from a previous study10, the clinical outcomes of deferred non-culprit lesions based on FFR and the comparative risk between deferred non-culprit lesions of ACS and deferred lesions in SCAD patients according to the FFR value have not yet been fully investigated.
Therefore, the current study investigated the clinical outcomes of deferred non-culprit lesions according to FFR in ACS patients and compared these with deferred lesions in SCAD patients.
Methods
STUDY DESIGN AND PATIENT POPULATION
The current study population was derived from the pooled population of the Korean 4-Centers registry and 3-vessel FFR FRIENDS study. The 4-Centers registry (NCT01409577) is a registry of patients who were treated using an FFR-guided strategy. From 2003 up to 2011, 1,294 consecutive patients (1,628 lesions) who underwent FFR measurement for at least one de novo lesion in a major epicardial vessel were enrolled from four major cardiovascular centres in Korea11. In ACS patients, the culprit lesions were revascularised without FFR measurement, followed by FFR measurement for the other non-culprit lesions. The 3-vessel FFR FRIENDS study (NCT01621438) is a prospective, multinational, multicentre study. From 2011 to 2014, 1,136 consecutive patients (3,298 lesions) who underwent FFR measurement for all three major epicardial vessels were enrolled from 11 centres in three countries12. In the 3-vessel FFR FRIENDS study, ACS patients also underwent revascularisation for clinically indicated culprit lesions, followed by FFR measurement for non-culprit lesions. In both registries, patients with depressed left ventricular systolic function (ejection fraction <35%), acute ST-elevation myocardial infarction (STEMI) within 72 hours, previous coronary artery bypass graft surgery (CABG), abnormal epicardial coronary flow (TIMI flow <3) or planned CABG after diagnostic angiography were excluded.
Among the total population of the two studies, 335 ACS patients whose non-culprit lesions were deferred based on an FFR >0.80, and 1,295 non-ACS patients with 2,484 deferred lesions based on an FFR >0.80, were extracted for the current analysis. Among ACS patients, 34 patients with unclear culprit lesions were excluded from the analysis.
DEFINITIONS OF ACUTE CORONARY SYNDROME
In the current study, ACS patients included those with non-ST-segment elevation myocardial infarction (NSTEMI), which was defined as a combination of clinical presentation, positive biomarkers with or without electrocardiographic changes, and those with unstable angina who had recent onset angina or accelerating or rest angina with electrocardiographic changes, but without evidence of positive biomarkers.
ANGIOGRAPHIC ANALYSIS AND QUANTITATIVE CORONARY ANGIOGRAPHY
Coronary angiography was performed using standard techniques. All angiograms were analysed at a core laboratory in a blinded fashion. Quantitative coronary angiography was performed in optimal projections with validated software (CAAS II; Pie Medical Imaging BV, Maastricht, the Netherlands). Minimum lumen diameter, reference vessel size and lesion length were measured, and percent diameter stenosis was calculated.
CORONARY PHYSIOLOGIC MEASUREMENTS
All coronary physiologic measurements were performed after diagnostic angiography. A 5-7 Fr guide catheter was used to engage the coronary artery, and a pressure-temperature sensor guidewire (St. Jude Medical, St. Paul, MN, USA) was used for FFR measurement. The pressure sensor was positioned at the distal segment of a target vessel, and intracoronary nitrate (100 or 200 mg) was administered before each FFR measurement. Hyperaemia was induced with an intracoronary bolus administration (80 μg in the left coronary artery, 40 μg in the right coronary artery), or an intracoronary (240 μg/min) or intravenous continuous infusion (140 μg/kg/min) of adenosine. FFR was calculated as the lowest average of hyperaemic proximal aortic pressure (Pa)/distal coronary arterial pressure (Pd) during three consecutive beats under stable hyperaemia. After FFR measurement, the guidewire was pulled back to the guide catheter, and the presence of pressure drift was checked.
PATIENT FOLLOW-UP, OUTCOME MEASUREMENTS AND ADJUDICATION OF CLINICAL EVENTS
Clinical data were obtained at outpatient clinic visits or by telephone contact when needed. An independent clinical events committee adjudicated all events. The primary outcome was major adverse cardiac events (MACE), including cardiac death, target vessel-related myocardial infarction (MI) and target vessel-related ischaemia-driven revascularisation. All clinical outcomes were defined according to the Academic Research Consortium criteria, including the addendum to the definition of MI. Ischaemia-driven revascularisation was defined as a revascularisation procedure with at least one of the following: (1) recurrence of angina; (2) positive non-invasive test; and (3) positive invasive physiologic test. Clinical outcome analysis was carried out during a median follow-up of 722.0 days (interquartile range 657.0-747.0).
STATISTICAL ANALYSIS
Data were analysed on a per-patient basis for clinical characteristics and on a per-lesion basis for the others. For per-lesion comparison of characteristics, a generalised estimating equation (GEE) was used to adjust intrasubject variability among deferred lesions from the same patient. The cumulative incidence of clinical events was presented as Kaplan-Meier estimates and compared using the log-rank test. The hazard ratio (HR) and 95% confidence intervals (CI) were calculated using a marginal Cox proportional hazards regression model in order to adjust the clustering of multiple vessel measurements in the same patient. In comparison of clinical events between ACS and SCAD, the differences in patient and lesion profiles were adjusted using a multivariable adjusted marginal Cox proportional hazards regression model. As a sensitivity analysis, comparison of clinical outcomes was also performed on a per-patient basis between ACS patients with deferred non-culprit lesions and SCAD patients with deferred lesions. For this, the differences in baseline characteristics were adjusted using a multivariable adjusted Cox proportional hazards regression model.
A multivariable marginal Cox model was used to identify independent predictors of MACE. C-statistics with 95% CI were calculated to validate the discriminant function of the model. All probability values were two-sided, and p-values <0.05 were considered statistically significant.
Results
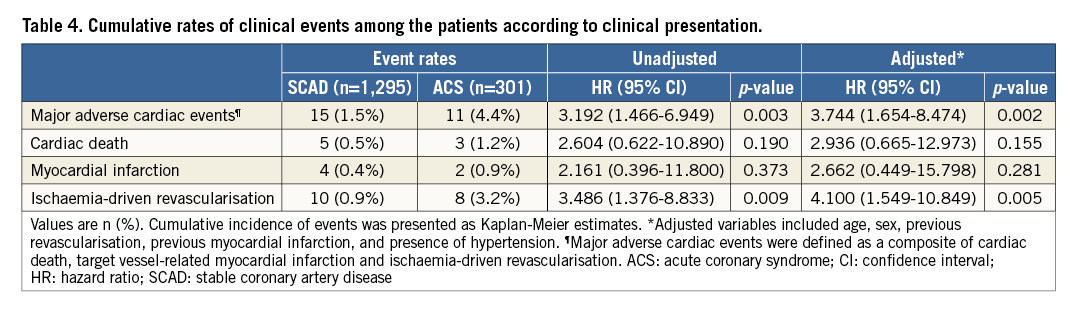
CHARACTERISTICS OF PATIENTS AND LESIONS
Table 1 presents baseline characteristics of the study population. Although distributions of cardiovascular risk factors were similar between SCAD and ACS patients, the SCAD population showed a higher proportion of hyperlipidaemia, previous MI, and previous revascularisation. Among the ACS population, 65.8% of patients presented with unstable angina and 34.2% with NSTEMI. In 2,933 deferred lesions, the mean percent diameter stenosis and FFR were 39.3±15.0% and 0.92±0.06, respectively. The deferred non-culprit lesions in ACS patients showed significantly higher stenosis severity and longer lesion length despite a similar FFR value to those in SCAD patients (Table 2). The distribution of FFR in deferred lesions was similar between SCAD and non-culprit vessels in ACS patients (Figure 1).
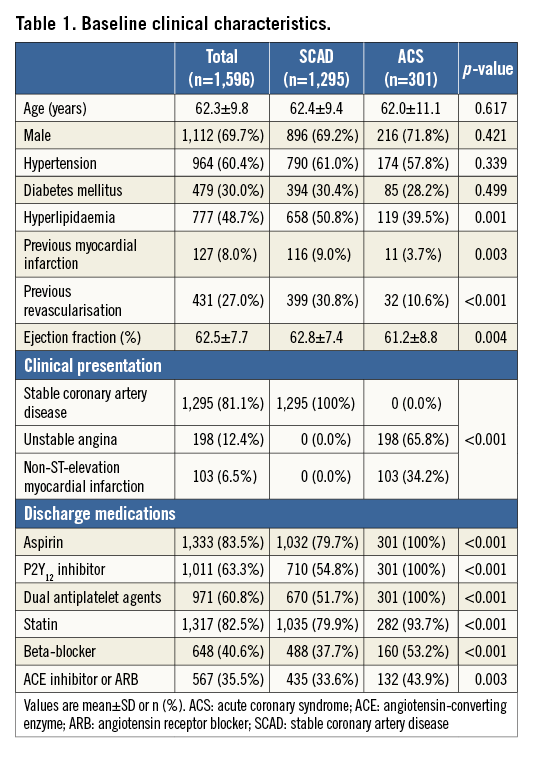
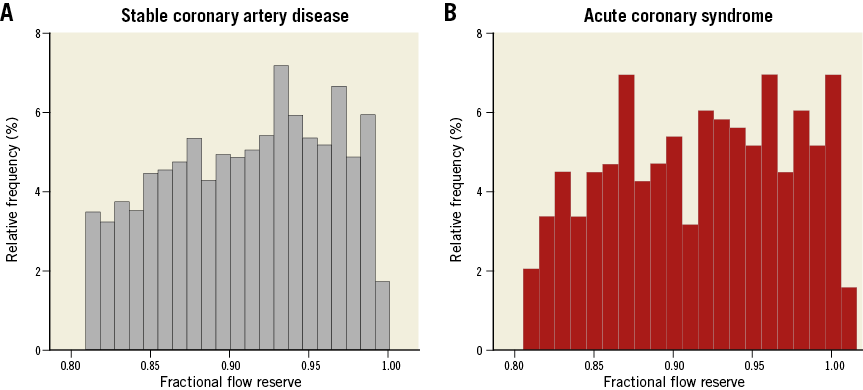
Figure 1. Distribution of fractional flow reserve in deferred lesions. Distribution of FFR values in (A) 2,484 deferred lesions in SCAD patients and (B) 449 deferred non-culprit lesions in ACS patients.
CLINICAL OUTCOMES OF DEFERRED LESIONS IN PATIENTS WITH ACS AND SCAD
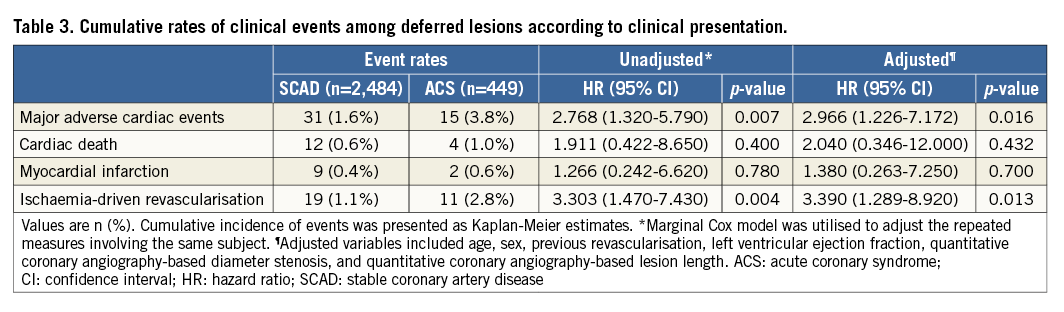
Deferred lesions in ACS patients showed a significantly higher rate of MACE compared with those in SCAD patients (3.8% vs. 1.6%, log-rank p=0.001). The median time to event for two-year MACE was 265.0 days (Q1-Q3, 154.0-543.0) and 361.0 days (Q1-Q3, 220.0-618.0) for deferred non-culprit lesions in ACS patients and deferred lesions in SCAD patients, respectively. Even with adjustment of stenosis severity, lesion length, and different distribution of risk factors, deferred non-culprit lesions in ACS patients showed about a threefold higher risk of MACE than SCAD (HRadj 2.97, 95% CI: 1.23-7.17, p=0.016) (Figure 2A). The higher risk of MACE in ACS patients was mainly driven by the higher rate of ischaemia-driven revascularisation (HRadj 3.39, 95% CI: 1.29-8.92, p=0.013) (Table 3). The 56.7% of ischaemia-driven revascularisations were due to ACS, and the others were due to deferred lesion progression with objective evidence of ischaemia. When stratifying ACS by unstable angina and NSTEMI, the risk of MACE was not different (Figure 2B).
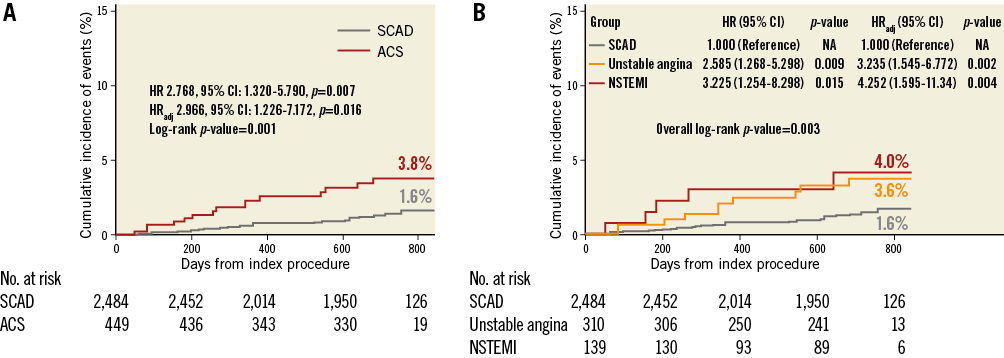
Figure 2. Comparison of MACE rates in deferred lesions between ACS and SCAD. Kaplan-Meier curves for the comparison of rates of MACE in deferred lesions according to (A) SCAD and ACS, (B) SCAD, unstable angina, and NSTEMI. ACS: acute coronary syndrome; CI: confidence interval; HR: hazard ratio; HRadj: multivariable adjusted hazard ratio; NSTEMI: non-ST-segment elevation myocardial infarction; SCAD: stable coronary artery disease
The patient-level comparison of MACE risk between ACS patients with deferred non-culprit lesions and SCAD patients with deferred lesions showed similar results to the lesion-level comparison (Figure 3, Table 4).
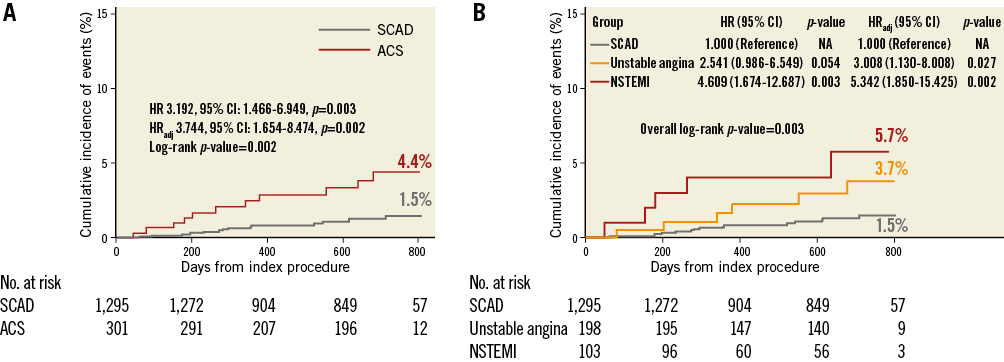
Figure 3. Patient-level comparison of MACE rates between ACS and SCAD. Kaplan-Meier curves for the comparison of rates of MACE (A) between ACS patients with deferred non-culprit lesions and SCAD patients with deferred lesions, and (B) according to clinical presentations (SCAD, unstable angina, and NSTEMI). ACS: acute coronary syndrome; CI: confidence interval; HR: hazard ratio; HRadj: multivariable adjusted hazard ratio; NSTEMI: non-ST-segment elevation myocardial infarction; SCAD: stable coronary artery disease
CLINICAL OUTCOMES OF DEFERRED LESIONS BETWEEN ACS AND SCAD ACCORDING TO FFR CATEGORIES AND IN SUBGROUPS
Figure 4 shows comparisons of the rates of MACE between the two groups according to FFR category. In all FFR categories, deferred lesions of ACS showed higher MACE rates than those of SCAD. The higher risk of MACE in deferred non-culprit lesions of ACS patients was similarly observed in various subgroups without significant interaction (Figure 5).
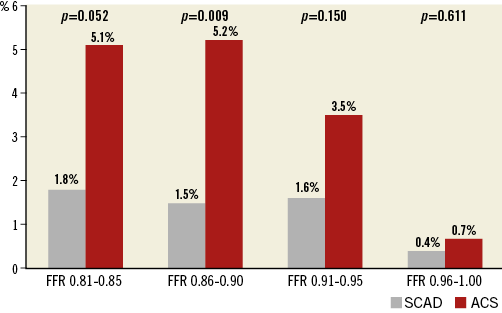
Figure 4. Comparison of MACE rates in deferred lesions between ACS and SCAD according to the range of FFR. The cumulative rates of MACE in deferred lesions were compared between SCAD and ACS, according to the range of FFR. ACS: acute coronary syndrome; SCAD: stable coronary artery disease
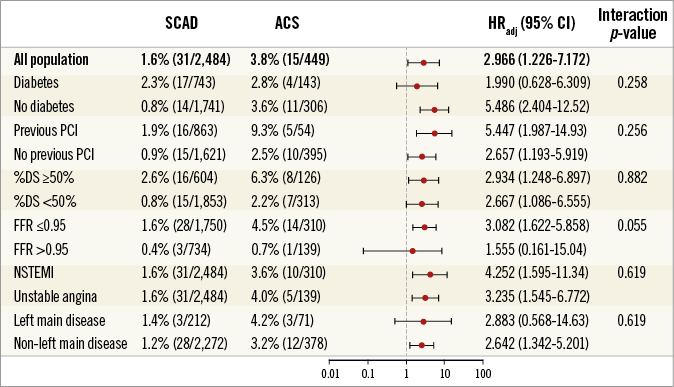
Figure 5. Comparison of MACE rates of deferred lesions between ACS and SCAD according to subgroup. The cumulative rates of MACE in deferred lesions were compared between SCAD and ACS, according to various subgroups. %DS: percent diameter stenosis; ACS: acute coronary syndrome; CI: confidence interval; HR: hazard ratio; HRadj: multivariable adjusted hazard ratio; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; SCAD: stable coronary artery disease
INDEPENDENT PREDICTORS OF MACE
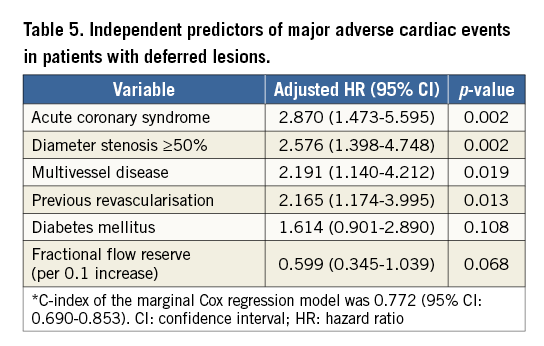
In a multivariable marginal Cox proportional hazard model of the total population, ACS was the most powerful independent predictor of MACE (HRadj 2.74, 95% CI: 1.13-6.64, p=0.026). Other independent predictors were diameter stenosis ≥50% and multivessel disease (Table 5). Among the deferred lesions with an FFR between 0.80 and 0.90, FFR value did not show significant association with the risk of MACE in both the ACS (HR of FFR per 0.1 increase 0.91, 95% CI: 0.09-9.56, p=0.934) and SCAD populations (HR of FFR per 0.1 increase 0.48, 95% CI: 0.09-2.59, p=0.395).
Discussion
The current study compared clinical outcomes between deferred non-culprit lesions in ACS patients and deferred lesions in SCAD patients based on FFR. Despite the similar FFR values in both groups, the rate of MACE was significantly higher in ACS patients than in SCAD patients. The higher risk of MACE in ACS patients compared to that in SCAD patients was consistently observed in all categories of non-ischaemic FFR values, as well as in various subgroups. Furthermore, in the multivariable predictive model, ACS was the most powerful independent predictor for MACE.
FFR-GUIDED STRATEGY IN ACS PATIENTS: PER-PATIENT LEVEL DECISION
The representative studies for per-patient level decision were the FAME ACS substudy3 and the FAMOUS-NSTEMI trial4. The FAME ACS substudy demonstrated the equivalent benefit of an FFR-guided strategy versus an angiography-guided strategy between ACS and SCAD populations. The FAMOUS-NSTEMI trial randomly allocated patients into an FFR-disclosure group versus an angiography-guided group and showed that the rate of revascularisation remained lower in the FFR-disclosure group with similar clinical outcomes and quality of life between the two groups4. Although these two studies showed clinical benefit of an FFR-guided strategy over an angiography-guided strategy in ACS patients, there has been no previous study which compared per-lesion-based clinical outcomes between ACS and SCAD patients, especially for deferred non-culprit lesions under the current FFR-guided strategy.
FFR-GUIDED STRATEGY IN ACS PATIENTS: PER-LESION LEVEL DECISION FOR CULPRIT LESIONS
Fischer et al presented comparable one-year clinical outcomes between 35 ACS patients and 76 SCAD patients whose revascularisation was deferred based on an FFR >0.75 (28.5% vs. 17.0%, p=0.21)5. Potvin et al also presented similar rates of cardiac events between 124 ACS patients and 77 SCAD patients whose revascularisation was deferred based on an FFR ≥0.75 at 24 months of follow-up (11% in ACS vs. 19% in SCAD, p=0.47)6. Although these relatively small studies reported comparable outcomes between culprit lesions in ACS patients and lesions in SCAD patients, recent studies have shown different results.
Mehta et al evaluated clinical outcomes of 334 ACS patients and 340 non-ACS patients whose revascularisation was deferred based on an FFR >0.80 (mean follow-up 4.5 years) and reported that deferred ACS patients had a higher risk of cardiac events (32% vs. 23%, p=0.02). In their study, a lower FFR value was associated with an increase in adverse cardiac events in the ACS group (HR 1.08 per 0.01 decrease in FFR, 95% CI: 1.03-1.12)7. In a study by Hakeem et al, 206 ACS patients with deferred culprit lesions based on an FFR >0.75 showed higher rates of MACE than 370 SCAD patients during a mean follow-up of 3.4 years (25% vs. 12%, p<0.001); the authors proposed a cut-off value of FFR <0.84 in the culprit vessel of ACS patients8. However, it is unclear whether these findings can be reproduced in deferred non-culprit lesions.
FFR-GUIDED STRATEGY IN ACS PATIENTS: PER-LESION LEVEL DECISION FOR NON-CULPRIT LESIONS
Previous studies focusing on the relevance of an FFR-guided per-patient level decision did not separately present the clinical outcomes according to culprit and non-culprit lesions. Furthermore, all the previous studies which evaluated the FFR-guided per-lesion level decision focused on the assessment of culprit lesions of ACS patients. The current study focused mainly on the clinical outcomes of deferred non-culprit lesions of ACS patients rather than the culprit lesion. In a comparison of lesion characteristics, deferred non-culprit lesions of ACS patients showed higher stenosis severity and longer lesion length than deferred lesions in SCAD. Nevertheless, the deferred non-culprit lesions in ACS patients showed a significantly higher risk of MACE than deferred lesions in SCAD patients, even after adjustment for baseline clinical and lesion differences. These results suggest that the plaque characteristics of non-culprit lesions in ACS patients might be a potential mechanism for the higher event rates.
The significant difference of two-year MACE rates between deferred non-culprit lesions in ACS patients and deferred lesions in SCAD patients was mainly driven by ischaemia-driven revascularisation. As the current study included deferred lesions with insignificant FFR values, the cumulative rate of death or MI was relatively low, as in previous studies with deferred lesions1,3. The higher risk of MACE in deferred non-culprit lesions of ACS rather than SCAD patients might be partly explained by the results from intravascular imaging studies which consistently presented the higher prevalence of vulnerable features in non-culprit lesions of ACS patients compared with lesions of SCAD patients13,14. The link between vulnerable plaque characteristics in non-culprit lesions of ACS patients and the risk of clinical events was also shown by those studies15.
In our study, the MACE rate was consistently higher in ACS patients than in SCAD patients in all FFR categories above 0.80 and in various subgroups. The absolute difference in MACE rate between ACS and SCAD in FFR ranges of 0.81-0.85 and 0.86-0.90 was similar (3.3% in FFR 0.81-0.85 vs. 3.7% in FFR 0.86-0.90). In addition, among the deferred lesions with FFR between 0.80 and 0.90, FFR value did not show a significant association with the risk of MACE in both ACS and SCAD populations. This finding is different from the results of studies by Hakeem et al8 and Mehta et al7, which assessed the outcome of FFR-guided deferral in culprit lesions for ACS, and presented a higher risk of MACE in the lower range of FFR value and suggested a higher FFR cut-off value in the culprit lesion evaluation. Theoretically, microvascular injury in patients with ACS can underestimate lesion severity, especially in the myocardial territory of a culprit vessel2. However, our study finding supports the use of the current cut-off value of FFR for non-culprit lesions in ACS patients. In addition, the recently published studies which investigated the outcomes of an FFR-guided strategy for non-culprit lesions also support the use of an FFR-guided revascularisation strategy for non-culprit lesions in patients with STEMI16,17.
CLINICAL IMPLICATIONS
Our study showed that deferred non-culprit lesions in ACS patients showed a higher event rate than deferred lesions in a SCAD setting. This result may represent the systemic nature and plaque characteristics of ACS, which is associated with higher event rates, in both culprit and non-culprit vessels. Therefore, strict medical treatment and secondary prevention for comorbidities are required for ACS patients with deferred non-culprit lesions.
Limitations
This study has some limitations. First, the current study was not a randomised comparison of clinical outcomes following FFR-guided deferral or revascularisation for non-culprit lesions of ACS patients. Second, the intravascular imaging study was not systematically performed; therefore, the current study could not directly compare plaque characteristics and vulnerability between deferred non-culprit lesions in ACS patients and deferred lesions in SCAD patients. The difference in plaque characteristics may be one of the main mechanisms for the higher event rate in ACS patients than that in SCAD patients. Third, although FFR measurement was routinely performed in consecutive patients during the enrolment period of both registries, patients with acute STEMI or cardiogenic shock were excluded. In addition, as the current study focused on deferred non-culprit lesions of ACS patients rather than the culprit lesion, the lesions were mostly of mild to moderate severity. Fourth, although all the medications prescribed at discharge were mandatorily collected, the medications during follow-up were not systematically collected. Fifth, the ACS population was relatively small. Last, various methods for hyperaemia induction were used in this study. However, each centre applied the same method, for both ACS and SCAD patients included from that centre.
Conclusions
Deferred non-culprit lesions of ACS patients on the basis of non-ischaemic FFR showed significantly higher rates of clinical events, compared with deferred lesions of SCAD patients. This finding was consistent in various FFR categories and subgroups.
| Impact on daily practice The current study compared clinical outcomes between deferred non-culprit lesions in ACS patients and deferred lesions in SCAD patients based on FFR. Despite the similar FFR values in both groups, the rate of MACE was significantly higher in ACS patients than that in SCAD patients. The higher risk of MACE in ACS patients than that in SCAD patients was consistently observed in all categories of non-ischaemic FFR values, as well as in various subgroups. These results suggest a systemically increased risk of clinical events in the ACS population. Therefore, strict medical treatment and secondary prevention for comorbidities are required for ACS patients with deferred non-culprit lesions. |
Funding
Part of this study was supported by St. Jude Medical. The company had no role in study design, conduct, data analysis or manuscript preparation.
Conflict of interest statement
B-K. Koo has received an institutional research grant from St. Jude Medical. The other authors have no conflicts of interest to declare.

