
Abstract
Aims: Intracoronary adenosine (ICA) yields similar fractional flow reserve (FFR) results to the “gold standard” of intravenous adenosine (IVA). Whether they have similar prognostic significance is unknown. We therefore sought to study the prognostic value of the route of adenosine administration for the measurement of FFR in deferred coronary lesions in a large, real-world cohort.
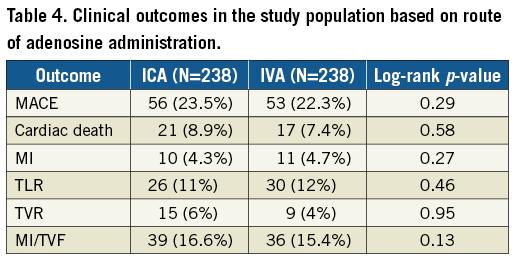
Methods and results: Five hundred and seventy-six patients with 787 lesions in whom PCI was deferred based on FFR >0.75 were studied. The primary outcome was the first major adverse cardiovascular event (MACE; defined as death, myocardial infarction [MI], or target vessel revascularisation [TVR]), and the secondary outcome was a composite of MI and target vessel failure (TVF). FFR was measured with ICA in 426 lesions and IVA in 361 lesions. Median follow-up duration was 3.2 years (interquartile range: 1.7-4.6). Propensity-matched cohorts of ICA and IVA were well matched for baseline clinical, angiographic and haemodynamic characteristics. In the propensity-matched cohort, MACE occurred in 23.5% of the ICA group and in 22.3% of the IVA group (p=0.29). On multivariate analysis, acute coronary syndrome, FFR and prior MI/revascularisation were independent predictors of MACE and MI/TVF. The route of adenosine administration was not predictive of MACE or MI/TVF.
Conclusions: ICA and IVA yield similar FFR values and show comparable long-term prognostic utility in a deferred population. These findings provide confirmation that non-ischaemic FFR using a simpler ICA protocol provides prognostic data similar to the gold standard IVA.
Abbreviations
FFR: fractional flow reserve
ICA: intracoronary adenosine
IQR: interquartile range
IVA: intravenous adenosine
MACE: major adverse cardiac events
MI: myocardial infarction
TVF: target vessel failure
Introduction
Fractional flow reserve (FFR) has become the standard of care in the assessment of the functional significance of coronary stenosis1. Fundamental to the accurate measurement of FFR is the achievement of maximal hyperaemia, defined as maximal vasodilation in the myocardial microvascular bed by more than twofold and the consequent severalfold increase in coronary blood flow. The pivotal FFR trials used intravenous adenosine administered through a central vein as the gold standard “agent”2-4. Other coronary vasodilators include intravenous or intracoronary nitroprusside, papaverine, nicorandil, and regadenoson. These agents were validated by several studies and may offer advantages including improved efficiency, ease of use, better side effect profile and possibly cost-effectiveness compared with the traditional gold standard without compromising the diagnostic efficacy of FFR evaluation5-7.
Given its ease of use and favourable safety profile, intracoronary adenosine (ICA) has long remained a very commonly used agent to achieve hyperaemia for FFR measurement. Multiple studies, including randomised trials, have shown a similar diagnostic accuracy of ICA for FFR measurement compared to the reference standard of IVA8-12. However, questions remain on the optimal dose of ICA required for maximal hyperaemia, with some suggesting that doses of up to 600 micrograms (μg) may be required to improve the diagnostic yield of FFR13. Similarly, concerns regarding suboptimal vasodilation and possible false negative results have led some to believe that higher doses of up to 180 µg/kg/min intravenously may be a more optimal intravenous adenosine dose14. Pitfalls in achieving maximal hyperaemia can lead to false negative results with potential untoward consequences, especially for deferred lesions. Whether the route of administration of adenosine for FFR measurement affects long-term prognosis remains unclear and has not been studied. Hence, we sought to study the prognostic value of the route of adenosine administration for the measurement of FFR in deferred coronary lesions in a large, real-world cohort.
Methods
STUDY POPULATION
Consecutive patients undergoing coronary angiography with measurement of FFR on at least one epicardial coronary artery from 2009 to 2014 at our institution were identified. All patients with an FFR >0.75 on whom coronary intervention was deferred were included in this analysis. The study was approved by the Central Arkansas Veterans Health System Institutional Review Board.
PROCEDURES
FFR measurements were performed using a 0.014” intracoronary pressure wire (PressureWire™ Aeris™; St. Jude Medical/Abbott, St. Paul, MN, USA, or PrimeWire®; Volcano [now Philips Healthcare, Amsterdam, the Netherlands]). After baseline Pd/Pa was obtained, intracoronary adenosine (ICA) or intravenous adenosine (IVA) was administered at the discretion of the operator. IVA was infused at a rate of ≥140 μg/kg/min through a peripheral intravenous line for at least 120 seconds. ICA protocol involved administration of ICA at a dose of at least ≥40 μg. When multiple measurements were made, the lowest achieved FFR was used.
DATA COLLECTION
For the included patients, we reviewed inpatient and outpatient electronic medical records in the Veterans Affairs Information Systems Technology and Architecture database (VistA). The Veterans Administration (VA), America’s largest integrated healthcare system, has a uniform, fully electronic national record system called the Computerized Patient Record System (CPRS). It provides networked, robust, and timely retrieval of remote site patient data, including clinic visits, emergency department visits, outpatient phone contacts and hospitalisation records. In the case of hospitalisations outside the VA, either they are recorded in physician notes or outside records are scanned and stored electronically in the CPRS. Manual extraction of patient information and records from the VistA/CPRS interface programme was performed blindly and independently by three investigators.
Data on demographics, comorbidities, prior coronary revascularisation, medications and indication for coronary angiography were obtained from review of the pre-catheterisation assessment record. Patients with unstable angina (UA) and non-ST-elevation myocardial infarction (NSTEMI) were grouped as acute coronary syndrome (ACS). Data on visually estimated angiographic stenosis, number of vessels involved, baseline Pd/Pa and FFR at maximal hyperaemia were collected.
ENDPOINTS
The primary study outcome was the occurrence of a major adverse cardiovascular event (MACE), which was defined as a combination of myocardial infarction (MI), target vessel revascularisation (TVR) or death. MI was defined as a rise in troponins with at least one value above the 99th percentile or upper limit of reference lab value along with symptoms and/or electrocardiographic or imaging evidence of ischaemia. TVR was defined as revascularisation of the coronary artery at the site of the original lesion or a site other than the index lesion assessed by FFR. The secondary endpoint was a combination of MI and target vessel failure (TVF; defined as TLR/TVR or MI related to the initially interrogated vessel with FFR). All patient events were censored at the occurrence of the first event. The date of occurrence of the primary endpoint or date of last clinic follow-up with either cardiology or primary care was used to determine follow-up duration. All events were adjudicated by a data review committee comprising two members.
STATISTICAL ANALYSIS
Baseline characteristics in the ICA and IVA groups were compared using the Student’s t-test for continuous variables and the chi-square test for categorical variables. We used propensity score matching to adjust for imbalances in key baseline characteristics between these two groups. Propensity score matching was performed using a non-parsimonious logistic regression model with ICA/IVA as the dependent variable, and the following as covariates: age, sex, diabetes mellitus, chronic kidney disease (CKD) on presentation, prior history of myocardial infarction or prior revascularisation, prior peripheral vascular disease (PVD) or stroke (CVA), presence of acute coronary syndrome (ACS) on presentation, and presence of multivessel disease. One-to-one propensity-score nearest neighbour matching without replacement was applied with a calliper of 0.2 to generate the two matched groups. Overall balance test showed that the data were balanced (p=0.54)15. Predictor variables were tested before and after matching to ensure balanced distribution. After propensity score matching, the baseline covariates were compared again between the two groups with a paired t-test or Wilcoxon signed-rank test for continuous variables and the McNemar test for categorical variables. Clinical outcomes were analysed using patient level analysis. Lesion characteristics, including FFR and haemodynamics, were analysed by lesion level analysis. As choice of route of administration and clinical variables may be clustered on patient (e.g., one route of adenosine for all lesions in a single patient with multivessel disease), multilevel modelling was used to account for this random effect. The p-values using Z-statistics are listed for individual covariates for their fixed effects.
A Kaplan-Meier survival analysis was used to estimate survival curves, and the log-rank test to test differences between groups. Multivariate models were constructed using a Cox proportional hazards model for MACE and MI/TVF prediction stratified on matched pairs. Backward stepwise elimination was used to eliminate non-significant variables to derive a final multivariate model (inclusion criteria p<0.05; exclusion criteria p>0.10). The number of covariates entered into the model was restricted to maintain ≥10 events per degree of freedom (i.e., one variable for 10 events). Known clinical predictors of MACE and univariate predictors with p<0.1 were entered into the model. Two-sided probability values with a p-value <0.05 were considered statistically significant. All statistical calculations were performed using MedCalc Statistical Software, version 15.2.1 (MedCalc Software, Ostend, Belgium), SPSS, Version 22 (IBM Corp., Armonk, NY, USA) and Stata 13 (StataCorp, College Station, TX, USA).
Results
We identified 576 patients and 787 individual coronary lesions that met our inclusion criteria. FFR was measured by administration of ICA in 426 lesions in 285 patients and by IVA in 361 lesions in 291 patients. Median follow-up duration was 3.2 years (IQR 1.7-4.6).
BASELINE CHARACTERISTICS
Baseline characteristics of the ICA and IVA groups in the original and propensity-matched cohort are shown in Table 1. Before matching, ICA patients had a significantly higher prevalence of chronic kidney disease (28% vs. 19%, p=0.012). The prevalence of a history of prior MI and/or coronary revascularisation was higher in the IVA group (69% vs. 57%, p=0.003). One third (33.5%) of all procedures were carried out for ACS, with a significantly higher proportion of ACS patients in the IVA group (43% vs. 29%, p=0.001). The use of beta-blockers and ACE inhibitors was similar. However, patients in the IVA group had a higher nitrate use (43% vs. 26%, p<0.05).

On propensity matching, each group comprised 238 patients. ICA and IVA propensity-matched groups were well matched for all baseline comorbidities and medications on deferral except for a higher proportion of patients on nitrates in the IVA group (Table 1, Table 2).

ANGIOGRAPHIC AND HAEMODYNAMIC CHARACTERISTICS
The procedural characteristics of the IC and IV groups are presented in Table 3. Angiographic stenosis was 48.7±12% in the ICA group and 53.8±13% in the IVA group (p=0.002). Mean baseline Pd/Pa and FFR were similar in both groups (Figure 1). The median dose of IC adenosine in our cohort was 130 (IQR: 120-216) μg, median dose in the RCA was 72 (IQR: 51-138) μg and 140 (IQR: 120-230) μg in the left coronary system. In addition, the proportion of patients in the ICA and IVA groups within a specific FFR category achieved was similar (Figure 2). There was no association between the FFR value obtained and route of adenosine administration (p=0.45). The distribution of individual vessels interrogated was as follows (ICA vs. IVA): left anterior descending artery (36% vs. 35%); left circumflex artery (27% vs. 23%); right coronary artery (21% vs. 24%), and left main artery (4.1% vs. 9%) (p=NS).

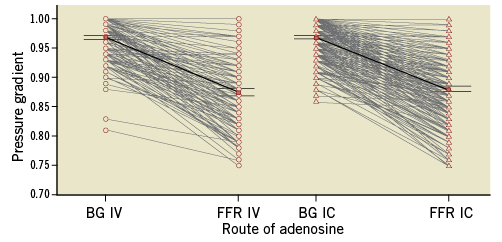
Figure 1. Comparison of degree of hyperaemia and FFR obtained by intravenous versus intracoronary adenosine. BG: baseline gradient
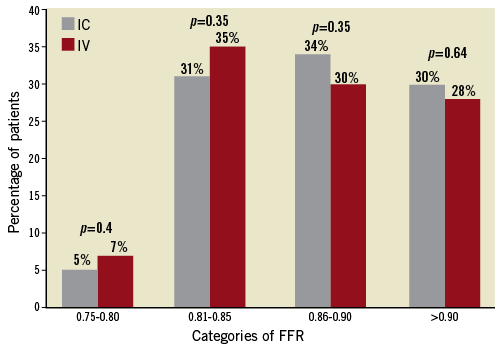
Figure 2. Proportion of patients categorised according to degree of maximal hyperaemia achieved by intravenous versus intracoronary adenosine (overall p=0.22).
CLINICAL OUTCOMES
Over a median follow-up of 3.2 years, MACE occurred in 23.5% of the ICA group compared with 22.3% in the IVA group (p=0.29) (Figure 3). There was no significant difference in MACE and secondary endpoints (cardiac death, MI, TLR, TVR, MI/TVF) between the two groups (Table 4).
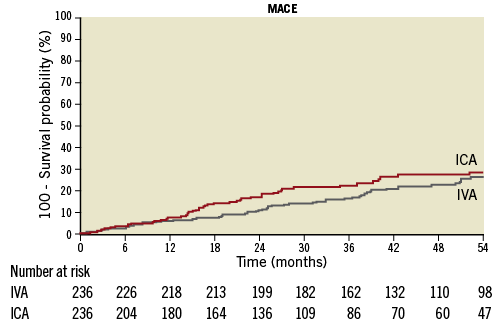
Figure 3. Kaplan-Meier curve for incidence of MACE based on IC/IV adenosine (log-rank p-value 0.24).
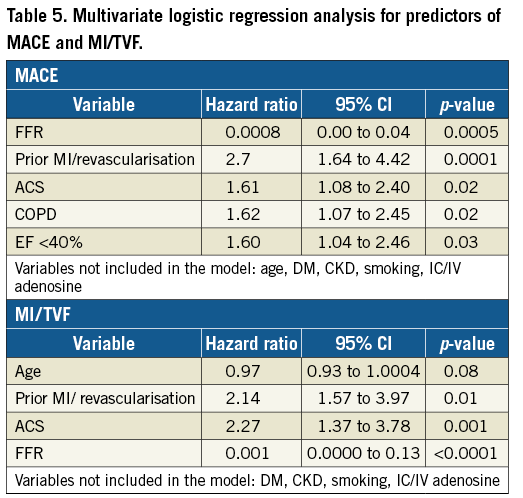
On multivariate analysis, ACS (HR 1.61, 1.08-2.40), history of MI/revascularisation (HR 2.70, 1.64-4.42), LVEF <40% (HR 1.60, 1.04-2.46) and COPD (HR 1.62, 1.07-2.45) were independent predictors of MACE (Table 5). ACS and prior MI/revascularisation were independent predictors of the secondary endpoint of MI/TVF (Table 5). The route of adenosine administration was rejected during the analysis as a predictor and hence not predictive of MACE or MI/TVR in the Cox regression models (Table 5).
Discussion
The main finding of this study is that the long-term prognosis of non-ischaemic FFR is independent of the route of adenosine administration. Furthermore, we showed that there was no association between the route of adenosine administration and the FFR values obtained. ACS presentation, FFR, COPD, LV dysfunction and previous history of MI or revascularisation were the only independent predictors of long-term MACE in patients with non-ischaemic FFR. To the best of our knowledge, this is the first study to compare the long-term prognostic utility of FFR obtained by the IC vs. the IV route.
FFR is the gold standard for assessing intermediate coronary lesions in the cardiac catheterisation laboratory and is being used increasingly1. It requires pharmacologically induced maximum hyperaemia in the myocardial microvascular bed, with adenosine being the most commonly used agent. Adenosine has a short half-life (less than 10 seconds) and is rapidly eliminated from the circulation by erythrocyte uptake and deamination. How it should be administered remains a topic of persistent debate. In the DEFER (Percutaneous Coronary Intervention of Functionally Nonsignificant Stenosis) trial, adenosine was administered either IV (140 μg/kg/min) or IC (15 μg in the right or 20 μg in the left coronary artery) with a cut-off FFR of 0.75 to denote ischaemia4. However, in the pivotal FAME (Fractional Flow Reserve-Guided PCI versus Medical Therapy in Stable Coronary Disease) and FAME 2 trials, only IV adenosine at a rate of 140 μg/kg/min was administered via a central vein with an FFR cut-off of 0.802,3.
COMPARISON OF FFR ACHIEVED BY IV VS. IC ADENOSINE
In this study, we demonstrated that FFR was independent of the route of adenosine administration. These findings are in agreement with previous retrospective studies comparing FFR obtained with IV vs. IC adenosine. Schlundt et al compared IC vs. IV adenosine administration in 114 patients and reported excellent FFR correlation (r=0.99; p<0.001) between these two groups8. The use of ICA was associated with improved patient comfort and reduced procedural time. Lopez Palop et al compared ICA bolus doses of 60, 180, 300, and 600 μg to IVA 140 μg/kg/min and 200 μg/kg/min and reported similar FFR values achieved9. A few studies have shown an incremental hyperaemic response to increasing doses of ICA that correlate with FFR obtained with IVA10-12. Other studies have also demonstrated an excellent correlation between FFR values obtained using ICA and IVA13-17.
PROGNOSTIC IMPACT OF FFR ACHIEVED BY IVA VS. ICA
We demonstrated, for the first time, the similar prognostic utility of FFR by ICA vs. IVA. Although ICA was used in the DEFER trial, doses used in current practice are much higher and have not been validated4. In addition, the impact of the route of adenosine administration on prognosis was not analysed in the DEFER study. Our results validate the prognostic value of non-ischaemic FFR obtained by ICA in a contemporary patient cohort in which ICA was used at doses comparable to current practice. A non-ischaemic FFR, regardless of the route of adenosine administration, was associated with similar long-term prognosis for individual and composite endpoints.
SELECTION OF IVA VS. ICA
Significant practice variations in terms of the route of adenosine administration exist in the interventional community. ICA administration is simple, convenient, cost-effective, and has fewer side effects of systemic hypotension and bronchospasm. Ntalianis et al reported that IVA was associated with longer procedural time than ICA (11 min vs. 9 min, p=0.04)18. Disadvantages of ICA include lack of dose recommendation, possibility of lack of vasodilation in the entire myocardial bed and short-lasting vasodilation which makes the timing of FFR measurement critical. Since recording of the aortic pressure has to be interrupted for ICA, administration of the medication has to be rapid. In addition, optimal engagement of the guide catheter to the coronary ostium is critical to ensure accurate drug delivery. IVA has the advantages of having a tested and safe protocol, weight-based dosing, the ability to cause prolonged vasodilation that allows multiple FFR measurements, and a pullback in patients with ostial disease, diffuse disease and multiple or serial lesions. However, IVA is expensive, uses a large amount of drug and is more resource-intensive19. Apart from practical concerns, ICA has also been thought to induce a lesser degree of vasodilation due to selective injection into a coronary artery, as opposed to IV adenosine where the entire myocardial bed becomes hyperaemic.
Although ICA administration may have some advantages, as previously noted, continuous IVA infusion allows more comprehensive haemodynamic evaluation of serial stenosis, ostial lesions and diffuse disease by pullback which is not possible with an ICA bolus19.
FFR is an invaluable tool in the current era of balancing clinical efficacy, safety and cost-effectiveness. Despite strong supporting evidence, its use has been disappointing. A one-year (2008 to 2009) analysis from the National Cardiovascular Data Registry showed that FFR is used in only 6% of intermediate lesions20. A potential barrier to widespread FFR is IVA which requires additional time, resources and personnel. Thus, the use of ICA has practical advantages. While previous studies have demonstrated equivalent (or at least non-inferior) diagnostic performance of ICA vs. IVA for FFR measurement, the prognostic value of ICA has thus far been presumed to parallel that of IVA but without validation. The results of this study demonstrated for the first time that the prognostic value of non-ischaemic FFR is similar using either route of adenosine administration. The fact that, with or without matching, the event rates were not higher in the ICA group is reassuring (Figure 3). In a nationwide analysis, we have recently shown that FFR use has increased 18-fold since publication of the FAME trial. However, there was a significant disparity in FFR utilisation between teaching and non-teaching hospitals, limiting its widespread use21. As the need for procedural efficiency keeps increasing, the use of a simpler intracoronary protocol could potentially help to minimise this disparity in FFR utilisation and increase widespread adoption into clinical practice. In an expert consensus statement on the standardisation of FFR reporting from various catheterisation laboratories, using IVA through a large vein to achieve maximal hyperaemia and to avoid additional ICA boluses was recommended22. A consensus statement from the Society of Cardiovascular Angiography and Intervention recommends the use of ICA as an alternative to IVA adenosine in patients with obstructive airway disease, but not on a routine basis23.
Limitations
Certain limitations merit consideration when interpreting our findings. This is a retrospective, single-centre study involving a referral population of USA veteran patients with higher cardiac risk factors and comorbidities which may limit the generalisability of our results to other patient groups24. Patients lost to follow-up or who had clinical events at other institutions may not have been accounted for. However, we expect the numbers involved to be low and affecting both groups similarly. In addition, patients in the IVA group had a higher prevalence of prior MI and coronary revascularisation and ACS presentations. However, this difference was accounted for with propensity score matching where the results were quite similar to the entire cohort. Data on adverse events such as heart blocks which could have impacted significantly on the dose of IC adenosine used during FFR measurement were unavailable.
Conclusions
In summary, we demonstrated similar prognostic utility of non-ischaemic FFR obtained by IV and IC adenosine. The route of adenosine administration does not affect the pressure gradient change across the lesion and is not an independent predictor of MACE, including MI/TVR. The routine use of IC adenosine, and the use of IV adenosine in selected cases, may be an acceptable strategy with practical advantages and could facilitate widespread FFR adoption.
| Impact on daily practice The use of higher doses of ICA than routinely used in current practice might provide similar FFR accuracy and prognosis, with IVA reserved for evaluation of diffuse and sequential coronary lesions. Results of this our current study provide reassurance for using ICA routinely and could help consolidate the role of ICA in FFR acquisition and facilitate increased clinical use of FFR in general. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

