
Abstract
Aims: The aim of this study was to evaluate the accuracy of a continuous intracoronary (IC) adenosine infusion, administered through the novel HYPEREM™IC over-the-wire microcatheter, to measure fractional flow reserve (FFR).
Methods and results: The HYPEREMIC trial was a randomised, non-inferiority, crossover study in which patients with intermediate coronary lesions were enrolled for sequential pressure wire studies. FFR was measured using intravenous (IV) (140-180 mcg/kg/min) versus continuous non-weight-adjusted IC (360 mcg/min) adenosine. Patients were randomised and blinded to the order in which they received the adenosine, separated by a washout period. The primary endpoint was the mean hyperaemic FFR. Forty-one patients were enrolled at three UK sites between June and November 2016. The mean (standard deviation) FFR was 0.82 (±0.09) after IC versus 0.84 (±0.09) after IV adenosine. The difference of –0.02 (95% confidence interval [CI]: –0.03 to –0.01) confirmed the non-inferiority (margin <0.05) of IC to IV adenosine. Intracoronary adenosine was associated with a shorter mean time to maximal hyperaemia (difference –44 [95% CI: –59 to –29] seconds; p<0.0001). Chest discomfort was reported in 32/41 (78.0%) patients during IV adenosine versus 12/41 (29.3%) patients during IC adenosine.
Conclusions: Continuous IC adenosine was a reliable, faster and better tolerated method of achieving maximal hyperaemia compared to IV adenosine.
Introduction
Pressure wire-based fractional flow reserve (FFR) has become the reference standard index of haemodynamic significance to guide revascularisation of intermediate coronary artery lesions1,2,3. Establishing maximal hyperaemia is essential for accurate FFR assessment4. Given the guideline-mandated thresholds for decision making, it is crucial that maximal hyperaemia be achieved in a reliable and reproducible manner. FFR measurements have historically been based on an intravenous (IV) adenosine infusion administered via a central venous catheter2. Although this approach allows a stable steady state to be achieved rapidly in most patients, it has largely been superseded by peripheral IV adenosine infusion, which affords similar FFR values and aligns with the increasing use of radial artery access for coronary intervention4,5.
Latterly, an intracoronary (IC) bolus of adenosine has gained widespread traction both for its simplicity and in response to the patient- and laboratory-level variability observed with IV administration. IC adenosine is better tolerated by the patient, obviates the need for extra venous access and allows repeat measurements to be conducted quickly6. An IC bolus, however, may require incremental doses of adenosine to achieve maximal hyperaemia, does not allow pullback measurements, and is inappropriate for the study of ostial lesions4. A continuous IC adenosine infusion can potentially overcome the drawbacks of bolus IC adenosine7. We therefore sought to determine whether hyperaemia induced by a continuous IC adenosine infusion, given through a novel microcatheter, would be non-inferior to the hyperaemia mediated by a standard IV protocol for the attainment of accurate FFR values.
Methods
The HYPEREMIC trial (ClinicalTrials.gov Identifier: NCT02527616) was a randomised, non-inferiority, single-blinded, crossover study designed to evaluate the utility and accuracy of a novel over-the-wire microcatheter (HYPEREM™IC; Diasolve Diagnostic Solutions, Salisbury, United Kingdom) to administer a non-weight-adjusted continuous IC adenosine infusion to achieve maximal hyperaemia for FFR measurement. The study was granted approval by the research ethics committee in Cambridge, UK, and by the Medicines and Healthcare Products Regulatory Agency (MHRA). The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
DEVICE DESCRIPTION
The HYPEREMIC microcatheter is an over-the-wire microcatheter with a proximal assembly designed to allow both insertion of a pressure wire and infusion of a hyperaemic agent simultaneously through the same central lumen (Figure 1). It has a 3 Fr outer diameter and is compatible with 6 Fr guide catheters and all commercially available pressure wires. The atraumatic radiopaque tip of the microcatheter can be placed a few millimetres into the artery under study without adversely influencing aortic or distal coronary pressure (Supplementary Appendix 1, Supplementary Figure 1).
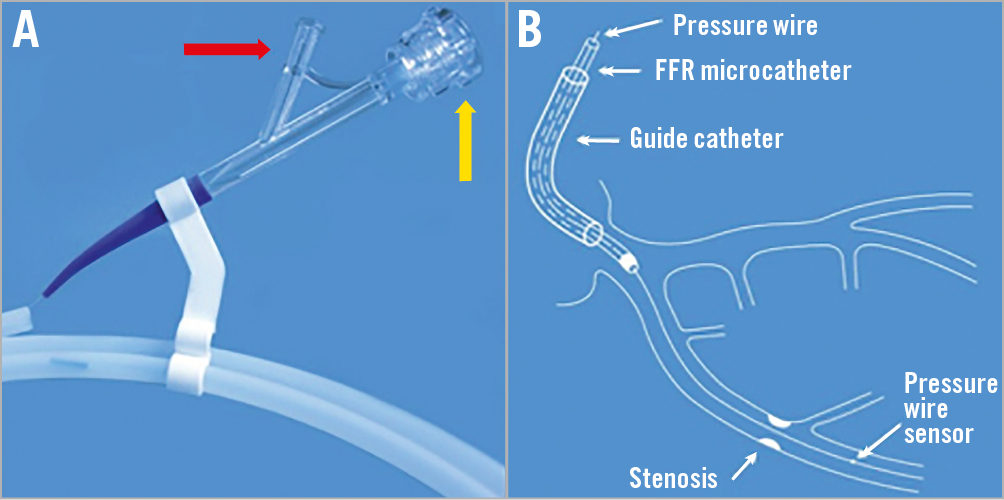
Figure 1. Procedural use of the HYPEREMIC microcatheter. The microcatheter is flushed with heparinised saline via the bifurcation luer of the two-arm adaptor (A: red arrow). Using a guidewire introducer, the pressure wire is then inserted into the microcatheter via the specific pressure wire port valve (A: yellow arrow). The pressure wire is advanced to the tip of the microcatheter. The microcatheter (plus pressure wire) is then loaded into the guide catheter via the haemostatic valve of a standard Y connector. The microcatheter is then advanced until the proximal marker band 1 (Supplementary Appendix 1) aligns with the haemostatic valve on the Y connector. This indicates that the microcatheter tip is approaching the guiding catheter tip. Under fluoroscopy, the radiopaque tip of the microcatheter is then advanced until it reaches the tip of the guide catheter. Bleed-back should be seen freely from the bifurcation luer of the two-arm adaptor (A: red arrow) to ensure that no air bubbles are trapped within the lumen. The infusion line from a standard infusion pump is connected and purged with the vasodilator (1 ml) solution. The pressure wire is then advanced to exit the guide tip and the microcatheter to the desired sensor position for equalisation according to standard manufacturer instructions (B). The pressure wire can then be advanced distal to the lesion or diseased segment under investigation as per standard FFR measurement protocol (B). The radiopaque tip of the microcatheter can then be advanced under fluoroscopy to the desired position within the artery under investigation but should remain proximal to the lesion or diseased segment. Extensive bench and animal testing (unpublished) has demonstrated that the microcatheter does not cause a significant pressure effect within a 6 Fr guiding catheter system or when introducing it in the proximal segment of the diseased artery to be interrogated. Several preclinical measurements with and without the catheter in place have shown that pressure measurements have not been affected by the microcatheter (see highlighted sections in Supplementary Appendix 2). Infusion of the vasodilator solution can then be commenced.
TRIAL DESIGN
Patients were enrolled at three centres in the United Kingdom (London, Glasgow and Brighton). All patients undergoing angiography, with a view to percutaneous coronary intervention (PCI) if mandated, were approached to enter the study. Eligible subjects had an angiographically identified intermediate coronary artery stenosis indicated for FFR assessment by standard criteria. Patients with acute ST-segment elevation myocardial infarction, haemodynamic instability, known hypersensitivity to adenosine or lesions deemed unsuitable for FFR measurement were excluded. Each trial participant gave written informed consent as approved by each institution.
Each patient received both IV and IC adenosine infusions, with the order determined by 1:1 randomisation in a blinded fashion. In effect, patients acted as their own control. The half-life of adenosine is <10 seconds; therefore, a three-minute washout period was instituted between each adenosine infusion to eliminate potential carryover effects from one treatment period to the next. Hyperaemia with IV adenosine was achieved with a standard infusion at 140-180 mcg/kg/min administered via a central or peripheral venous cannula according to operator discretion.
Hyperaemia with IC adenosine was achieved with a non-weight-adjusted infusion at 360 mcg/min administered via the microcatheter. The decision to use this dose for continuous IC infusion was derived primarily from an investigation by Yoon and colleagues, who used a different microcatheter and pressure wire assembly7. Animal testing with different IC infusion doses was also performed prior to this human study (Supplementary Appendix 2). Randomisation was performed using a validated online tool (www.sealedenvelope.com).
In those lesions found to be haemodynamically significant, operators removed the complete microcatheter system, including pressure wire, to enable use of their preferred workhorse coronary guidewire to perform PCI. In practice, however, only the microcatheter need be removed using standard techniques, leaving the pressure wire in the coronary artery to perform PCI.
HYPOTHESIS AND ENDPOINTS
The study hypothesis was that the mean hyperaemic FFR value achieved with IC adenosine would be non-inferior (as low or lower) compared with that achieved by IV adenosine. The primary endpoint was the mean hyperaemic FFR measured in the distal coronary artery, beyond the intermediate coronary lesion under investigation, recorded after a steady state of maximal hyperaemia had been reached. Secondary endpoints included the incidence and severity of side effects, time to and dose of adenosine required to achieve maximal hyperaemia. Subjects were asked during and after each pressure wire sequence to grade verbally any symptoms of chest discomfort that they experienced. Other periprocedural and post-procedural adverse events were recorded.
STATISTICAL ANALYSIS
The non-inferiority margin was set at 0.05 based on previous acceptable limits8. The sample size calculation assumed an expected FFR of 0.77 after IV and an FFR of 0.74 after IC adenosine, with a common standard deviation of 0.107. Within-subject correlation between the two modes of administration was estimated conservatively at 0.95. With a one-sided 2.5% alpha level, 90% power could be achieved with 30 subjects. To allow for some degree of deviation from these initial sample size assumptions and a potential 10% drop-out rate, we planned to recruit 44 subjects. Period effects were deemed unlikely due to the washout period and short half-life of adenosine but if a period by treatment interaction were to occur then this sample size would still have provided 79% power to test the trial hypothesis.
All statistical analyses were performed using SAS version 9.3 or higher (SAS Institute Inc., Cary, NC, USA) based on a one-sided 2.5% alpha level. Three analysis populations were defined – the intention-to-treat (ITT) population consisting of all subjects randomised, the full analysis set (FAS) consisting of all subjects satisfying eligibility criteria and with data for both adenosine administration groups, and the safety population consisting of all patients commencing any procedure with either protocol, analysed according to the route by which adenosine was received. The primary endpoint analysis was assessed in both the FAS and ITT populations. By excluding protocol violators, the FAS population may be the more conservative for a non-inferiority comparison but is susceptible to bias in either direction. The safety analysis population was used for the principal assessment of side effects and adverse events.
For each subject the difference in FFR (FFRDIFF) was calculated as the FFR achieved during maximal steady-state hyperaemia with IC minus IV adenosine. This mean intra-patient difference with its standard deviation and two-sided 95% confidence interval was reported. The IC adenosine arm would be considered non-inferior to the IV adenosine arm if the upper limit of the two-sided 95% confidence interval for FFRDIFF was <0.05. This analysis assumed no order or treatment by sequence interaction effects. These assumptions were tested using independent t-tests9. If there were evidence of an interaction effect, then only the first sequence would be used in a between-patient analysis. Safety endpoints relative to each infusion were summarised according to their occurrence during the IC versus IV infusions and expressed as the number and percentage of subjects in each category by way of a descriptive summary rather than a formal analysis.
Results
A total of 41 patients were enrolled in the HYPEREMIC trial between June and November 2016 at three sites (Table 1). All subjects received their procedures in the correct randomised order, and all sequences were separated by the three-minute washout interval (Figure 2). No patients withdrew prematurely from the study. Two subjects failed to reach maximal hyperaemia during one of their procedural runs (one in each of the randomised groups) and were therefore removed from the FAS population. These two subjects remained in the ITT and safety populations. Eleven patients received an IV dose of 180 mcg/kg/min and the remainder received a dose of 140 mcg/kg/min, based purely on operator preference. A total of 38 (93%) patients had a peripheral IV, whereas three (7%) subjects received a central venous infusion of adenosine. For the IC arm, all 41 patients received the 360 mcg/min adenosine infusion via the microcatheter7. Procedures were performed via the right radial artery in 28 (68.3%), the left radial artery in 3 (7.3%) and the right femoral artery in 10 (24.4%) subjects. All procedures were completed using 6 Fr guiding catheters.
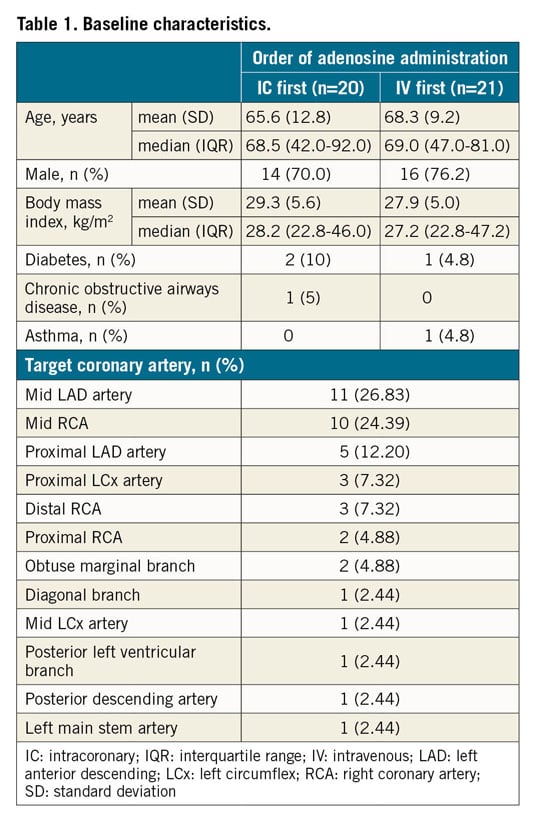
Figure 2. HYPEREMIC trial CONSORT diagram.
PRIMARY ENDPOINT
The mean (SD) FFR achieved was 0.82 (±0.09) by the continuous IC adenosine infusion and 0.84 (±0.09) via the IV adenosine infusion (Table 2). There was no evidence of a period effect (p=0.90) or a period/procedure interaction (p=0.75). The mean FFRDIFF was –0.02 (95% confidence interval [CI]: –0.03 to –0.01), thus confirming non-inferiority of continuous IC adenosine administered via the HYPEREMIC microcatheter versus a standard IV protocol for accurately measuring FFR (Figure 3). Five of 39 patients (12.8%) were classified as non-ischaemic by IV adenosine (FFR >0.80) but were found to be ischaemic by IC adenosine (FFR ≤0.80). No patients with a functionally ischaemic lesion according to IV adenosine were classified as non-ischaemic by IC adenosine.
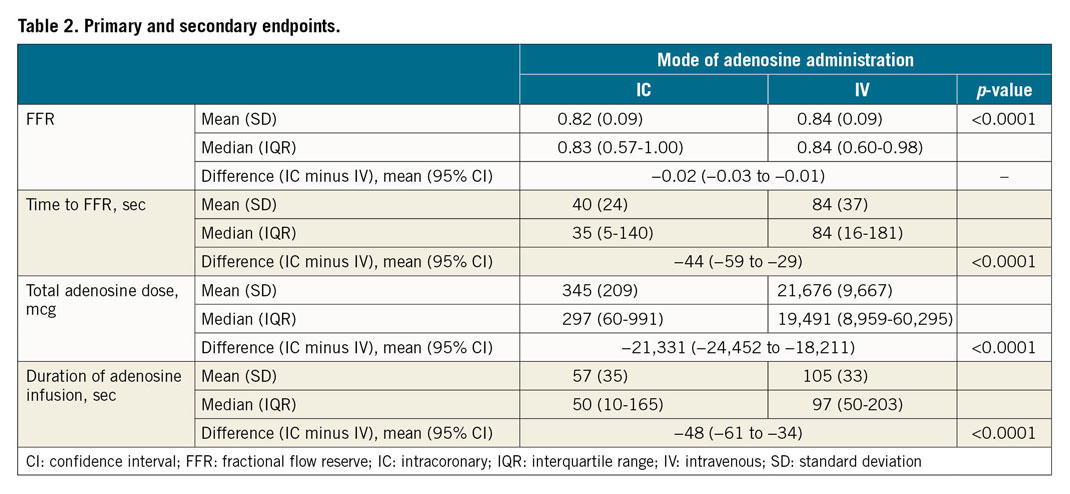
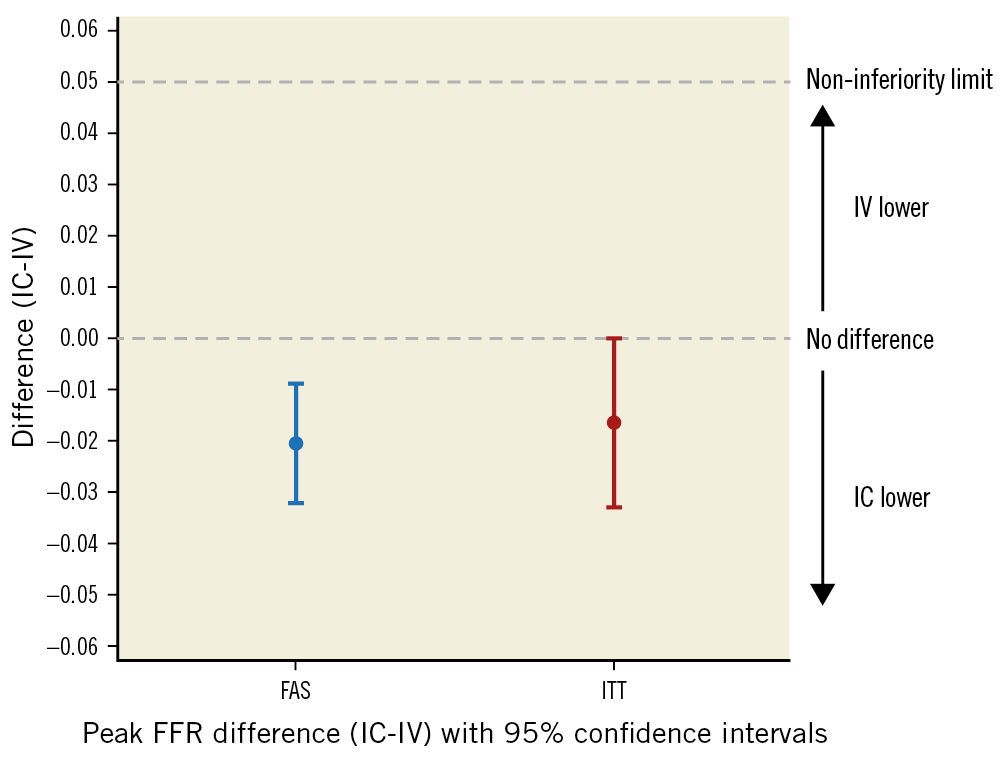
Figure 3. Primary endpoint assessment in the full analysis set (FAS) and intention-to-treat (ITT) populations. The upper confidence interval was lower than the pre-specified non-inferiority margin of +0.05 in both populations, indicating that intracoronary (IC) infusion was non-inferior to intravenous (IV) infusion for achievement of maximal hyperaemia. In addition, the upper confidence interval was less than zero, indicating that hyperaemia achieved by IC adenosine infusion resulted in a lower mean FFR than with IV adenosine.
TIME TO REACH MAXIMAL HYPERAEMIA AND DURATION OF ADENOSINE INFUSION
The IC infusion required a shorter mean time to achieve maximal hyperaemia (difference –44 [95% CI: –59 to –29] seconds; p<0.0001) (Table 2, Figure 4), and a shorter mean total duration of infusion (difference –48 [95% CI: –61 to –34] seconds; p<0.0001) (Figure 5).
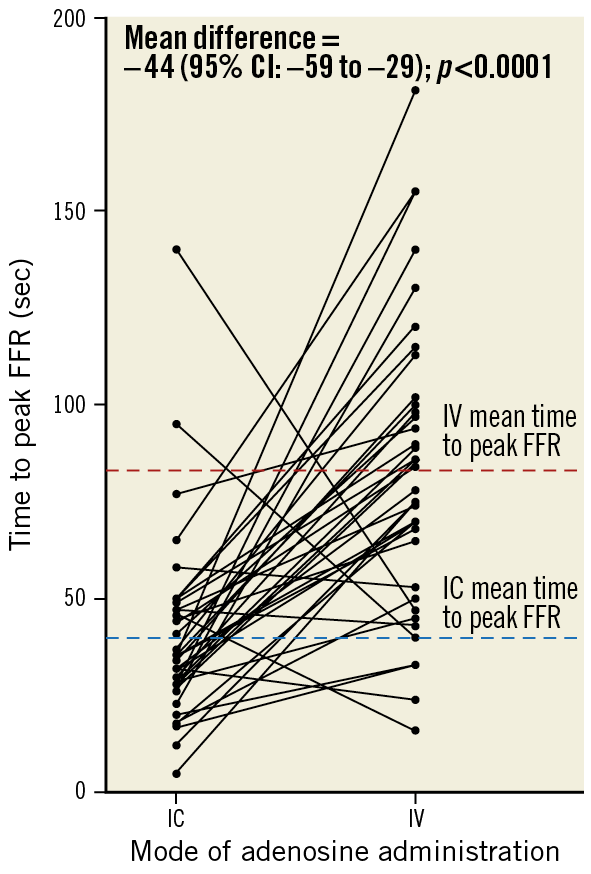
Figure 4. Mean time in seconds to the nadir of the FFR. CI: confidence interval; FFR: fractional flow reserve; IC: intracoronary; IV: intravenous
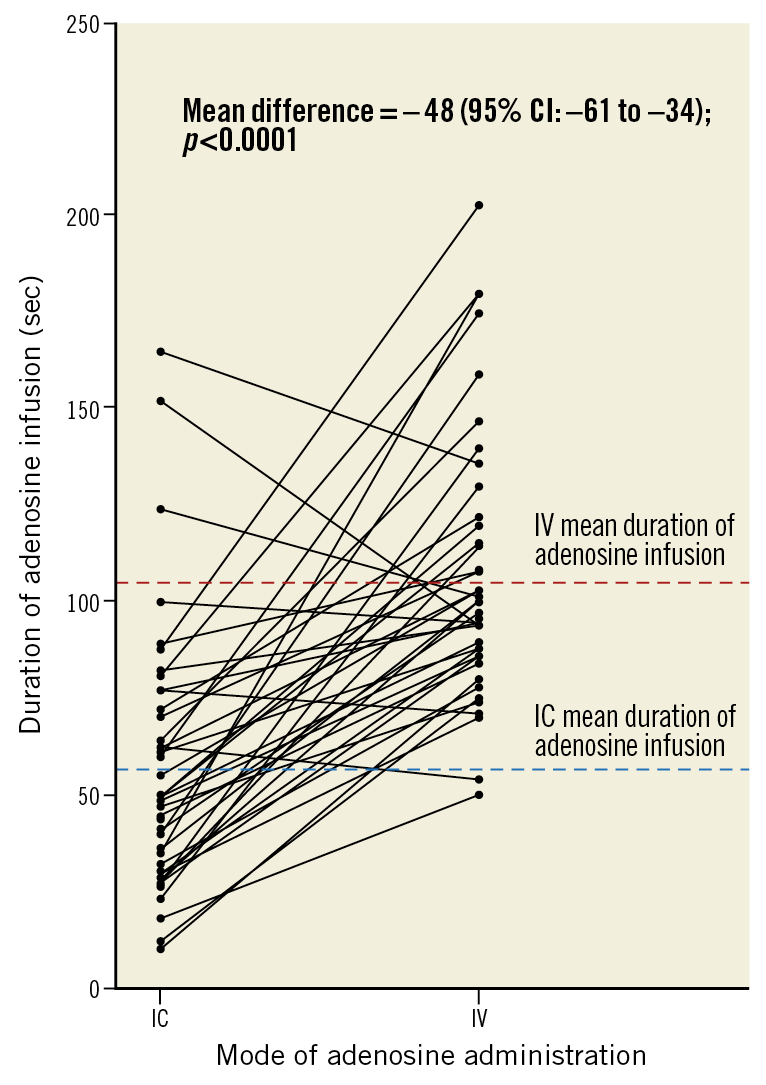
Figure 5. Mean duration of adenosine infusion. CI: confidence interval; IC: intracoronary; IV: intravenous
TOTAL ADENOSINE DOSE
IC infusion was associated with a lower total mean dose of adenosine required to induce maximal hyperaemia (difference –21,331 [95% CI: –24,452 to –18,211] mcg; p<0.0001) (Table 2).
SIDE EFFECTS
Chest discomfort of any severity was reported in 32/41 (78.0%) patients during IV infusions compared with 12/41 (29.3%) patients during IC infusions (Figure 6). No patients reported severe chest discomfort during IC infusion compared to 5/41 (12.2%) during IV infusion. The rate and severity of chest discomfort during and after IV adenosine was similar regardless of whether the IV infusion was the first or second procedure in the sequence.
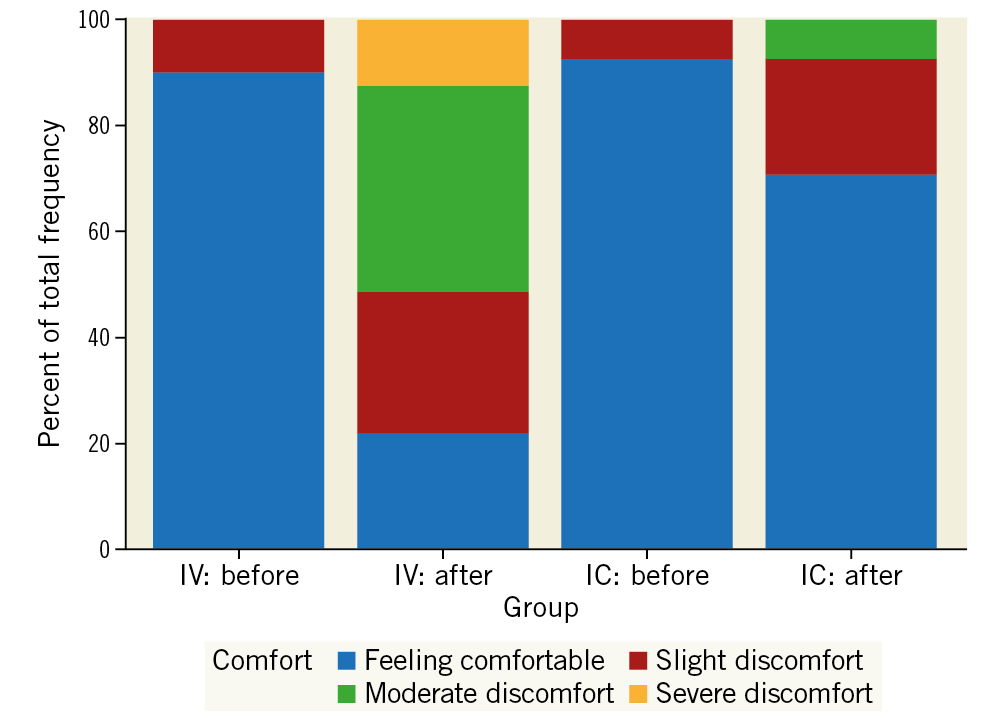
Figure 6. Chest discomfort recorded before and after each administration of adenosine. No patient experienced severe chest discomfort after an intracoronary (IC) infusion. The majority of patients (76%) felt comfortable after IC adenosine, whereas the majority of patients (78%) felt some degree of chest discomfort after intravenous adenosine. Chest discomfort was set as an exploratory categorical variable in the original statistical analysis plan.
Bradycardia was reported for one patient during their IV procedure (their second in the sequence). Four subjects had transient third-degree atrioventricular block reported (two during IC and two during IV procedures). Three of these were during the second procedure in the sequence. Haemodynamic indices recorded after intracoronary and intravenous administration of adenosine in the HYPEREMIC trial are shown in Supplementary Table 1.
Discussion
Contemporary surveys indicate that a significant proportion of interventional cardiologists do not routinely perform FFR to support their decision-making process10. The reasons for this are multifactorial: increased costs and lack of reimbursement predominate, alongside the extra time required for and side effects associated with central venous administration. For these reasons, operators moved to peripheral IV infusions4,5. Whilst faster and safer, patient- and laboratory-level variability persisted4,11. IC bolus adenosine requires a lower total dose of drug and is associated with fewer side effects, minimal haemodynamic disturbance, improved test/retest concordance, and general equivalence to FFR measurements acquired by an IV infusion4,8. Coronary artery-specific IC bolus dosing regimens that induce maximal hyperaemia with a minimal side-effect profile have been confirmed6. Steady-state hyperaemia, however, is short-lived with IC bolus adenosine, precluding pullback assessments of serial stenoses or diffuse disease. Evaluating ostial lesions may not be possible with IC bolus adenosine given the risk of plaque disruption or pressure damping during guide catheter engagement to ensure adequate distal drug delivery8.
A continuous IC infusion of adenosine may maintain the advantages of IC bolus adenosine while overcoming most if its drawbacks. Yoon and colleagues first investigated this concept by using a microcatheter that was introduced into the coronary ostium via a guide catheter. A separate externally calibrated pressure wire, sitting adjacent to the microcatheter within the same guide catheter, was then passed distal to the coronary lesion of interest7. Despite this burdensome set-up, a continuous IC adenosine infusion was shown to be safe, effective and quicker at achieving maximal hyperaemia than with IV adenosine, although the mean FFR values achieved were slightly lower.
Building on from this, the HYPEREMIC trial employed a dedicated over-the-wire microcatheter to administer a non-weight-adjusted continuous IC infusion of adenosine to achieve maximal hyperaemia. This device allows the pressure wire to be housed within the microcatheter rather than outside it. A continuous IC infusion using this microcatheter resulted in a slightly lower mean FFR compared with a standard IV adenosine protocol. The physical presence of the catheter itself could possibly explain this. However, the difference was just 0.02, and in 5/41 cases (12.2%) the ≤0.80 decision threshold was reached with IC but not with IV adenosine, and in four of these the FFR by IV adenosine assessment was <0.83, a nearly abnormal result within the error of the test. No subjects with a functionally ischaemic lesion (FFR ≤0.80) were classified incorrectly by IC infusion. This could have wide-ranging implications with regard to subsequent revascularisation decision making and raise important questions as to whether standard IV adenosine may be failing to achieve sufficient maximal hyperaemia in a proportion of subjects undergoing functional assessment of intermediate coronary artery stenoses.
IC compared with IV adenosine infusion also resulted in a significantly shorter time to attain maximal hyperaemia (more than twice as fast), and a shorter total duration of drug delivery. These outcomes could potentially result in significant time savings for operating staff and patients, thereby maximising workflow efficiency through the catheterisation laboratory.
The total average dose of adenosine administered to achieve maximal hyperaemia was significantly lower when using the IC protocol. In concert with the much lower baseline IC dosage, this resulted in the total hyperaemic drug delivery being ~1.5% of that given intravenously, a marked reduction which could translate into meaningful fiscal savings.
IC adenosine infusion was notably better tolerated by patients, with less chest pain compared with IV infusion. Furthermore, no subjects reported severe chest discomfort during and after IC adenosine infusion. Chest discomfort, however, was used as an exploratory categorical endpoint, and thus only a descriptive summary has been provided. Due to the crossover nature of the trial and the categorical nature of the endpoints, it was felt this would be the method providing most clarity. Interpretation should thus be based on clinical judgement of the expected/acceptable levels of chest discomfort.
The trial should be placed into context given the evolving landscape of physiological lesion assessment. The use of contrast medium to induce hyperaemia may be an option for centres in which adenosine is not easily available or very expensive, or in cases of adenosine hypersensitivity. Contrast FFR (cFFR) has demonstrated approximately 85% diagnostic performance against adenosine-based FFR12. The accuracy of cFFR in left main or proximal left anterior descending artery lesions may not, however, be as good as a standard hyperaemic adenosine protocol, presumably due to the large area of myocardium subtended by such lesions13. The evolution of non-hyperaemic methods of assessing functional impact should also be borne in mind. Resting Pd/Pa suffers from some of the same drawbacks as cFFR12,13. Instantaneous wave-free ratio (iFR), however, has been shown to be non-inferior to hyperaemia-induced FFR, whilst avoiding all of the potential adverse effects associated with adenosine14. Nonetheless, correlation of iFR with hyperaemic FFR is not perfect, and both still play an important role in assessing the borderline coronary artery lesion in accordance with site-specific protocols and operator preference.
Limitations
The number of lesions studied in the HYPEREMIC trial was small. Ostial and serial stenoses were not specifically studied, although a continuous IC infusion via the HYPEREMIC microcatheter can theoretically evaluate these lesion subsets. In ostial lesions, for example, positioning the microcatheter at the tip of the guide produced a similar hyperaemic effect without significant lag in hyperaemia compared to positioning the microcatheter in the proximal segment of the artery. This has been demonstrated in our own animal and bench testing (Supplementary Appendix 2). Beat-to-beat tracing analysis showing the transition to hyperaemia was not available for analysis.
Total costs were not collected, and therefore the trade-off for IC adenosine in reducing drug costs versus an additional cost for the microcatheter and performance of five extra PCI procedures were not ascertained. Finally, hard clinical endpoints were not assessed in the present study. Results from the HYPEREMIC trial warrant a larger clinical outcomes study comparing continuous IC adenosine infusion versus IV adenosine, bolus IC adenosine and/or iFR lesion assessment, inclusive of both ostial and serial coronary artery stenoses.
Conclusions
The utility of FFR to aid decision making for the management of intermediate coronary artery lesions is well established. The HYPEREMIC trial has demonstrated that a continuous infusion of a fixed dose of adenosine through a dedicated microcatheter effectively establishes maximal hyperaemia, with mean FFR values slightly lower than those achieved by a standard IV adenosine infusion, and results in a substantially faster and better tolerated procedure (for the patient) with a markedly lower total dose of adenosine required.
|
Impact on daily practice Continuous intracoronary adenosine given via a dedicated over-the-wire microcatheter is non-inferior to standard intravenous adenosine for the accurate assessment of FFR. Continuous intracoronary adenosine achieved maximal hyperaemia significantly faster, caused less chest pain and used approximately 1.5% of the dose of a standard intravenous adenosine infusion, which could translate into meaningful time and cost savings for catheterisation laboratories that continue to use adenosine-based pressure wire studies. A standardised intracoronary adenosine infusion given via a dedicated microcatheter could increase the uptake of guideline-mandated pressure wire studies to guide revascularisation of intermediate coronary artery lesions. |
Funding
The trial was fully sponsored by Diasolve Diagnostic Solutions, 114 Tetricus Science Park, DSTL Porton Down, Salisbury, SP4 0JQ, UK. The funders were involved in the design of the study. The funders were not involved in the collection, analysis and interpretation of the data. The funders were not involved in the preparation, review or approval of the manuscript.
Conflict of interest statement
A. Elghamaz is a medical advisor for the sponsor. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

