
Abstract
Aims: Our aim was to compare functional assessment of coronary stenosis severity by fractional flow reserve (FFR) measurement, as induced by systemic adenosine, and by regional reactive myocardial hyperaemia.
Methods and results: The primary study endpoints were coronary pressure-derived FFR values in response to intravenous adenosine infusion (140 µg/min/kg), and to a one-minute proximal coronary artery balloon occlusion (reactive hyperaemia) for the same stenosis of interest. The secondary study endpoint was coronary collateral flow index (CFI) during the same occlusion. CFI is the ratio between simultaneous mean arterial occlusive pressure and mean aortic pressure, both subtracted by central venous pressure. As a reference, coronary artery stenoses were assessed quantitatively as percent diameter reduction (%S). One hundred and twenty-five patients with coronary artery disease were included in the study. There was an inverse association between quantitatively determined structural stenosis severity and adenosine-induced FFR as well as post-ischaemic reactive hyperaemia FFR (%S=1-0.004 FFR; both at p<0.0001). Sensitivity and specificity for detecting a stenosis of ≥50% at an FFR threshold of 0.80 was 0.891 and 0.605 (adenosine-induced FFR), and 0.817 and 0.684 (post-ischaemic FFR), respectively. The FFR difference for a given stenosis (post-ischaemic minus adenosine-induced FFR) was directly related to CFI.
Conclusions: Regional reactive hyperaemia FFR is not inferior to systemic adenosine FFR in detecting structurally relevant coronary stenosis. Depending on the absence or presence of functional collaterals, systemic adenosine-induced FFR may underestimate or overestimate stenosis severity, respectively.
Introduction
In stable coronary artery disease (CAD), outcome after percutaneous coronary intervention (PCI) of an atherosclerotic stenosis is dependent on the amount of myocardial ischaemia1. Ischaemia is, among other things, influenced by the tightness and proximity of the lesion. Invasive ischaemia testing is relevant, because only a minority of patients with stable CAD undergo non-invasive examination prior to elective PCI2. Myocardial ischaemia assessment guides ad hoc PCI or its deferral, the latter aiming to prevent the adverse effects of a PCI, which would be ineffective on symptoms in the absence of ischaemia. Ischaemia testing in the presence of an intermediate coronary stenosis requires myocardial hyperaemia for assessing functional stenosis relevance. Hyperaemia should be maximal for accurate ischaemia testing and, during an invasive procedure, it is easily but variably provoked by adenosine3,4. Reasons for inconsistent, submaximal hyperaemia response to adenosine are its erratic dose response depending on the route of administration (intracoronary, intravenous peripheral, intravenous central), the changeable site response in the right versus left coronary artery, the absence or presence of coronary microvascular dysfunction (as in hypertensive or diabetic heart disease), and the occurrence of systemic haemodynamic side effects which prevent systematic peak dosing5. In addition, the degree of hyperaemia response is a function of collateral supply to a vascular area downstream of a coronary stenotic lesion. Hence, distal hyperaemic coronary pressure may be entirely normal despite the upstream presence of a tight stenosis, and distal pressures obtained during complete ostial occlusion may falsely indicate just a mild stenosis in case of well-developed collaterals from the contralateral side (Figure 1, Figure 2).
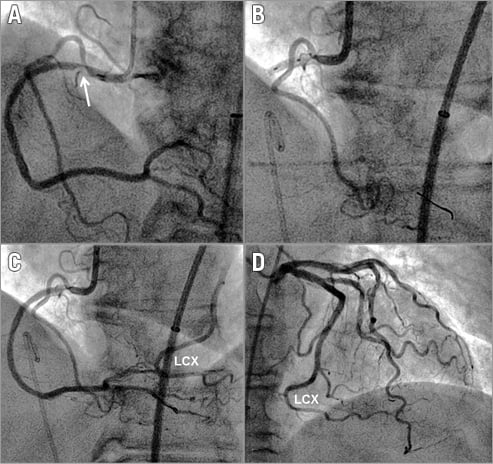
Figure 1. RCA collateral supply from the left circumflex artery. A) Angiogram of the right coronary artery (RCA) depicting an ostial atherosclerotic stenosis (arrow). B) Angioplasty balloon occlusion immediately downstream of the RCA lesion with imaging of the proximal right ventricular branch already shown in panel A. An angioplasty pressure sensor guidewire is positioned in the ramus interventricularis posterior (opaque tip). C) RCA angiogram immediately following release of the proximal angioplasty balloon occlusion as shown in panel B. The mid and distal part of the left circumflex artery (LCX) is also shown. D) Left coronary angiogram of the same patient with the identical mid and distal part of the LCX as depicted in panel C.
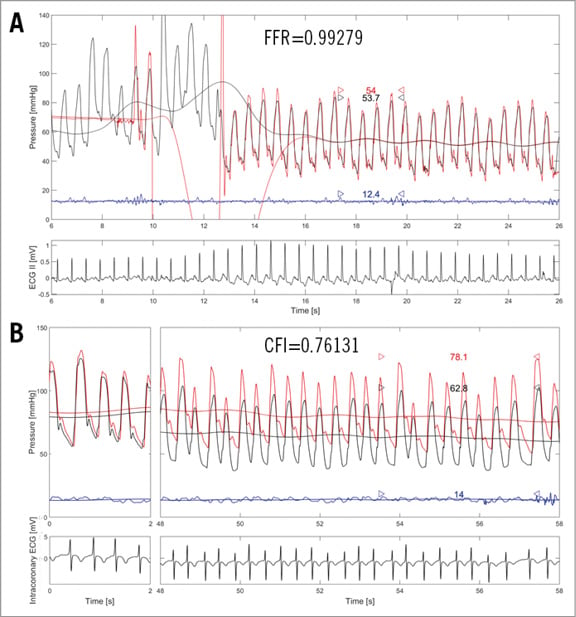
Figure 2. RCA collateral supply from the left circumflex artery: coronary functional measurements. A) Intravenous adenosine-induced fractional flow reserve (FFR) measurement as taken in the right coronary artery (RCA) of the patient shown in Figure 1. Distal coronary pressure (red phasic and mean curve, Pd) is obtained with the pressure wire positioned in the ramus interventricularis posterior (as shown in Figure 1B and Figure 1C); FFR =Pd/Pao (Pao: mean aortic pressure, black curve). The blue curve is central venous pressure (CVP) as obtained from the right atrium (not employed for FFR). B) Collateral flow index (CFI) measurement as obtained from the same patient during proximal RCA balloon occlusion (Figure 1B). CFI=(Poccl-CVP)/(Pao-CVP); where Poccl is Pd during coronary occlusion.
In this context, the present study tested the hypotheses that regional post-ischaemic reactive hyperaemia is equivalent in detecting structurally relevant coronary stenoses when compared to systemic adenosine-induced hyperaemia, and that the difference in functional stenosis relevance derived from the two methods of hyperaemia induction is related to coronary collateral function in the respective vascular region.
Methods
STUDY DESIGN AND PATIENTS
This was a prospective observational study in 125 patients undergoing coronary angiography for diagnostic purposes in the context of chest pain. The primary study endpoints were coronary pressure-derived fractional flow reserve (FFR) values as obtained in response to central intravenous (i.v.) adenosine infusion6,7, or to a one-minute proximal coronary artery balloon occlusion (reactive, post-ischaemic hyperaemia) from the same patient and for the same stenosis of interest: the occlusion was not performed within but outside the stenosis later to be treated by percutaneous coronary intervention (PCI)8. The secondary study endpoint was coronary collateral flow index (CFI) as obtained during the previously mentioned one-minute proximal coronary artery balloon occlusion for reactive hyperaemia induction9. Criteria for study inclusion were age >18 years, written informed consent to participate in the study, and 0- to 3-vessel chronic stable CAD. Exclusion criteria were acute coronary syndrome, previous myocardial infarction in the vascular region undergoing CFI measurement, and severe hepatic or renal failure (creatinine clearance <15 ml/min/1.73 m2). The study was approved by the Ethics Committee of the Canton of Bern, Switzerland, and all patients gave written informed consent to participate.
CARDIAC CATHETERISATION AND CORONARY ANGIOGRAPHY
Patients underwent left heart catheterisation and coronary angiography for diagnostic purposes from the right femoral artery approach via a 6 Fr introducer sheath. Biplane left ventriculography was performed followed by coronary angiography. Coronary artery stenoses were assessed quantitatively as percent diameter reduction using the guiding catheter for calibration. Aortic pressure (Pao) was obtained via a 6 Fr guiding catheter. Central venous pressure (CVP) was measured as right atrial pressure via the right femoral vein.
STUDY ENDPOINTS
The primary study endpoints were adenosine-induced and reactive hyperaemia FFR, which were calculated as mean distal coronary pressure divided by mean aortic pressure (Pd/Pao) (Figure 3). With that goal, a 0.014-inch pressure sensor angioplasty guidewire (PressureWire™; St. Jude Medical, St. Paul, MN, USA) was calibrated, advanced through and just outside the guiding catheter, where equalisation with the guide catheter pressure was performed. The sensor wire was then positioned in the distal part of the artery of interest.
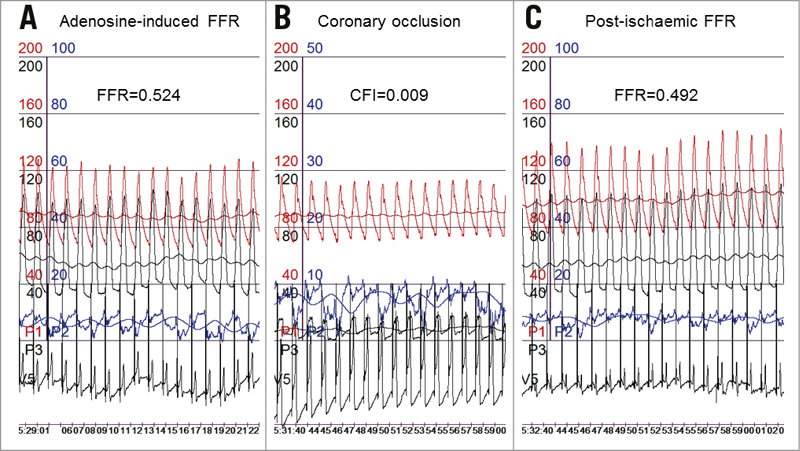
Figure 3. Coronary functional measurements. Measurements from the same patient and coronary stenosis of intravenous adenosine-induced fractional flow reserve (FFR) (A), of collateral flow index (CFI during a one-minute coronary balloon occlusion) (B), and of reactive hyperaemia, post-ischaemic FFR (C). FFR and CFI calculation as described for Figure 2. Red curves: mean and phasic aortic pressure; black pressure curves: mean and phasic coronary pressure; blue curves: mean and phasic central venous pressure. V5 ECG lead depicts the intracoronary ECG as taken from the angioplasty pressure guidewire.
The secondary study endpoint was CFI as determined by simultaneous measurement of mean aortic pressure (Pao, mmHg), the distal arterial pressure during balloon occlusion (Poccl, mmHg), and the central venous pressure (CVP, mmHg) (Figure 3) as obtained during the last 30 seconds of the one-minute proximal arterial balloon occlusion meant to induce post-ischaemic reactive hyperaemia. CFI was calculated as (Poccl-CVP) divided by (Pao-CVP)9. The accuracy of pressure-derived coronary CFI measurements in comparison to ECG signs of myocardial ischaemia during occlusion and to absolute myocardial perfusion measurements has been documented previously9,10.
The study comparator or reference parameter chosen was a comparator independent of the haemodynamic study endpoints: the structural coronary angiographic parameter of quantitative arterial percent diameter narrowing (% diameter stenosis).
STUDY PROTOCOL
Before the diagnostic exam, two puffs of oral isosorbiddinitrate were given. Following diagnostic coronary angiography and at the start of the invasive study procedure, all patients received 10,000 units of i.v. heparin. The coronary artery undergoing FFR and CFI measurements was chosen on the basis of the presence of a stenotic lesion requiring PCI or of ease of access in case of a non-stenotic vessel. Adenosine-induced FFR was always obtained prior to CFI. It was determined with the pressure guidewire positioned distally in the non-occluded coronary artery of interest using i.v. adenosine at 140 µg/kg/min for hyperaemia induction. Adenosine was infused for the duration of one minute via the side arm of a 6 Fr right femoral vein introducer sheath, while at the same time a 5 Fr pigtail catheter was positioned in the right atrium for CVP measurement (only employed for CFI calculation). Following adenosine-induced FFR measurement, a five-minute interval was allowed for complete hyperaemia dissipation. Meanwhile, and for the rest of the protocol, the pressure wire position was kept unchanged. For coronary CFI measurement, an adequately sized monorail angioplasty balloon catheter (diameters ranging from 2.5 to 5 mm) was positioned in the proximal part of the vessel and inflated at 1-2 atmospheres. Diagnostic one-minute coronary artery balloon occlusions at the previously mentioned inflation pressure in angiographically normal vessel segments have been documented to be safe8. Complete vessel occlusion was established angiographically and occlusion was maintained for exactly one minute. During vessel occlusion, simultaneous Poccl, Pao and CVP were obtained for the calculation of CFI (Figure 3). Right atrial pressure was taken as CVP via the 5 Fr pigtail catheter, as indicated above. Immediately following release of the proximal balloon occlusion for coronary CFI measurement, the angioplasty balloon was pulled back far into the guiding catheter and a coronary angiogram was performed. At the same time and immediately thereafter, simultaneous pressure monitoring of Pao and Pd (including CVP) continued for the calculation of post-ischaemic reactive hyperaemia FFR (Figure 3). If indicated, PCI of coronary atherosclerotic lesions occurred afterwards.
STATISTICAL ANALYSIS
For the purpose of data presentation, two study groups were formed based on the absence or presence of percent diameter coronary artery stenosis <50% or ≥50% as determined quantitatively in the vessel undergoing CFI measurement. Between-group comparison of continuous demographic, clinical, angiographic, haemodynamic variables, FFR and CFI data was performed by an unpaired Student’s t-test. A chi² test was used for comparison of categorical variables between the study groups. Intra-individual linear regression analysis was employed for univariate association testing between structural and functional stenosis severity data, and between FFR differences (post-ischaemic minus adenosine-induced FFR) and CFI. Receiver operating characteristic (ROC) curve analysis was used for assessing the accuracy of detecting a structurally relevant stenosis according to the above definition by adenosine-induced or by post-ischaemic FFR. Statistical significance was defined at a p-level of <0.05. Continuous variables are given as mean and standard deviation.
Results
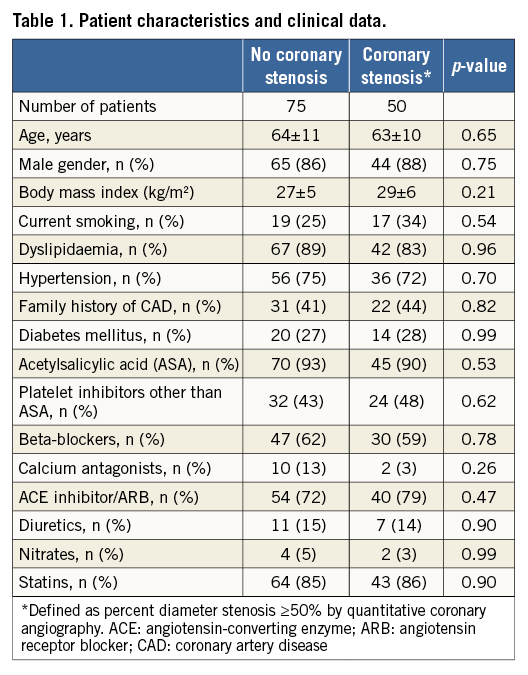
Seventy-five patients formed the group without structural coronary artery stenosis, i.e., with lesions <50% in diameter, and 50 patients were in the group with at least one stenosis (the lesion of interest) ≥50% in diameter narrowing (Table 1). In the context of the diagnostic proximal coronary artery balloon occlusion, no adverse events were observed either during hospitalisation or afterwards (no myocardial infarction, no deaths, no hospitalisation for acute coronary syndrome or new-onset angina pectoris).
PATIENT CHARACTERISTICS AND CLINICAL DATA
There were no statistically significant differences between the groups regarding age, gender, body mass index, cardiovascular risk factor prevalence, and cardiovascular medication (Table 1).
CORONARY ANGIOGRAPHIC AND HAEMODYNAMIC DATA
The number of coronary arteries with stenosis ≥50% in diameter (number of diseased vessels) was similar between the groups (Table 2). There was no significant difference between the groups in the prevalence of coronary arteries chosen for functional haemodynamic measurements (FFR and CFI). As per group definition, percent diameter stenosis was lower in the group without stenosis than in the group with stenosis (Table 2). FFR values irrespective of hyperaemia induction were higher in the former than in the latter group. CFI was lower in the group without than with stenosis (Table 2). Cardiac and coronary haemodynamic data were similar between the study groups.
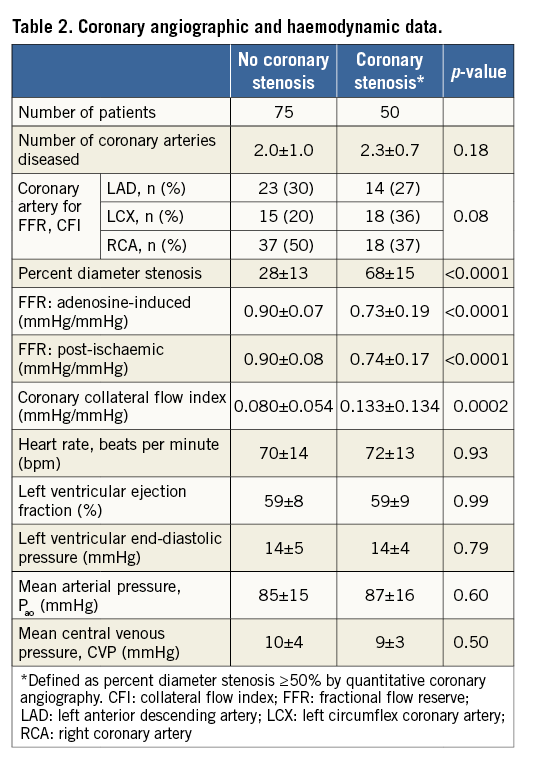
FUNCTIONAL STENOSIS SEVERITY AND ADENOSINE- INDUCED VERSUS POST-ISCHAEMIC REACTIVE HYPERAEMIA
There was a direct linear relation between systemic adenosine-induced FFR and regional post-ischaemic reactive hyperaemia FFR, whereby the latter was equal to 0.11+0.87 times adenosine-induced FFR, r2=0.0.814, p<0.0001 (Figure 4). There was an inverse association between quantitatively determined structural coronary stenosis severity (Figure 5, horizontal axes) and systemic adenosine-induced FFR (Figure 5A) as well as regional post-ischaemic reactive hyperaemia FFR (Figure 5B). The linear regression equation was identical between the two forms of hyperaemia induction, whereby a 50% stenosis corresponded to an FFR of 0.80; the squared regression coefficient was slightly lower using adenosine-induced than post-ischaemic hyperaemia. Adenosine-induced and post-ischaemic FFR detected a structurally relevant stenosis with an area under the ROC curve (AUC) of 0.777 and 0.829, respectively (95% confidence intervals of 0.669-0.885 and of 0.734-0.905, respectively; AUC difference =0.043; p=0.18 according to the DeLong test). Sensitivity and specificity for detecting a structurally relevant stenosis according to the group definition at an FFR threshold of 0.80 was 0.891 and 0.605 (adenosine-induced FFR), and 0.817 and 0.684 (post-ischaemic FFR), respectively.
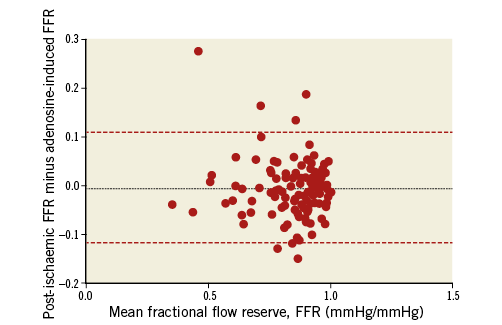
Figure 4. Bland-Altman plot of post-ischaemic and adenosine-induced FFR.
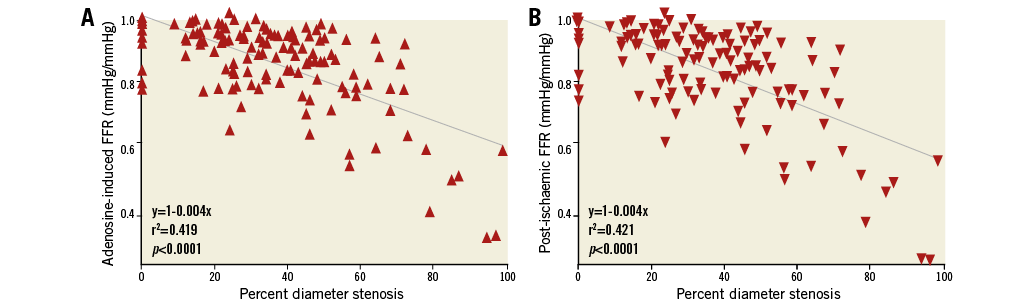
Figure 5. Coronary functional vs. structural stenosis severity. A) Correlation between percent diameter stenosis of the atherosclerotic lesion of interest and the respective intravenous adenosine-induced fractional flow reserve (FFR). B) Correlation between percent diameter stenosis of the atherosclerotic lesion of interest and reactive hyperaemia, post-ischaemic FFR.
There was a direct relation between the intra-individual difference in FFR (post-ischaemic minus adenosine-induced FFR) and coronary collateral function (CFI) (Figure 6).
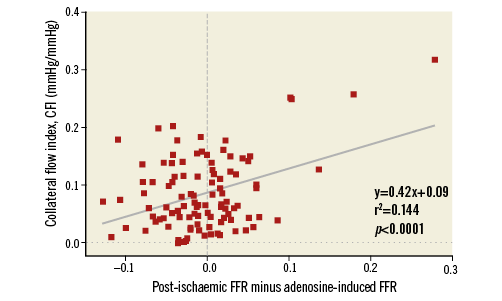
Figure 6. Correlation between reactive hyperaemia, post-ischaemic minus adenosine-induced fractional flow reserve (FFR) of the same atherosclerotic lesion and collateral flow index as obtained during proximal coronary occlusion in the same vessel (CFI).
Discussion
This study documented non-inferiority in detecting structurally relevant coronary stenosis by FFR in response to post-ischaemic reactive hyperaemia versus i.v. adenosine. I.v. adenosine underestimated functional coronary stenosis severity in the absence of well-developed collaterals, whereas in their presence functional stenosis severity was overestimated by systemic adenosine-induced versus regional reactive myocardial hyperaemia.
MYOCARDIAL HYPERAEMIA FOR FUNCTIONAL STENOSIS ASSESSMENT
Myocardial oxygen demand is determined by heart rate, ventricular wall stress, muscle shortening and contractility11. Since in the coronary circulation oxygen extraction from haemoglobin is already maximal at rest, myocardial oxygen supply is regulated exclusively by coronary flow11. Coronary flow at rest is autoregulated, that is, it remains constant at 1 ml/min/g of myocardium over a range of mean perfusion pressures between 60 and up to 140 mmHg12. As a direct consequence, it has been experimentally, and –in the end– also clinically demonstrated, that coronary atherosclerotic stenoses remain undetected up to 80% in diameter reduction, while even mild stenoses are uncovered during hyperaemia13,14. Functional assessment of stenosis severity during hyperaemia is obtained as capacity for maximal flow augmentation, i.e., as impaired flow reserve with resting flow in the numerator (for fractional flow reserve) or denominator (for coronary flow reserve). In the clinical invasive setting, coronary volume flow rate (Q) is difficult to obtain, and therefore surrogates of flow taken by angioplasty sensor guidewires, such as Doppler-derived flow velocity, transit time of a cold volume bolus (obtained by thermistor sensors) and distal coronary pressure are determined5. Among these parameters, coronary pressure measurements are least affected by artefacts or altering sensor positions, and thus represent the most robust of coronary haemodynamic invasive parameters5. Hence, it is coronary pressure-derived fractional flow reserve using adenosine for hyperaemia induction which underwent the most extensive, though not ideal, clinical evaluation15. In a pre-selected group of CAD patients “tagged” at the discretion of the operator to undergo stent implantation, subsequent randomised allocation to FFR-guided PCI (vs. no guidance) resulted in improved prognosis15. Thus, in the absence of a fair evaluation of structural (QCA) as compared to functional (FFR) stenosis assessment, but in need of a comparator for the present study, quantitatively obtained percent diameter narrowing of the stenosis was chosen as independent reference. The variability of percent diameter stenosis is explained for the most part by parameters other than altering FFR values, and this is irrespective of the type of hyperaemia induction (Figure 5). This data noise is probably related to insufficient coding of structural stenosis severity by the mere diameter reduction instead of also accounting for other, functionally relevant geometric features, such as stenosis length, shape, stenosis entrance and exit angle16.
THE CONDITION OF CONSTANT MAXIMAL HYPERAEMIA
Functional stenosis severity is unreliably represented by any of the parameters requiring myocardial hyperaemia, because the key conditions of constant and maximal microvascular resistance reduction are often not fulfilled. Specifically in the context of FFR, maximal, and thus constant hyperaemia is necessary so that coronary flow changes, which are impractical to obtain invasively, are directly reflected by coronary pressure variations6. In the present study, hyperaemia was achieved by systemic adenosine or by post-ischaemic, i.e., post-occlusive, reactive hyperaemia. The former was systematically applied according to conventional criteria7, thereby omitting the widely varying doses and dose responses of intracoronary adenosine3. Post-occlusive reactive hyperaemia was introduced more than 40 years ago as a very strong hyperaemic stimulus resulting in a fourfold to fivefold coronary blood flow increase in the canine heart17. Post-occlusive reactive hyperaemia is thought to result from the integrated effects of metabolically mediated vasodilation due to low pH, low partial oxygen tension, metabolic products, potassium flux, prostacyclin, and adenosine release from the interstitium18. In humans, post-occlusive reactive hyperaemia for haemodynamic stenosis assessment has only been tested while at the same time dilating the stenosis19. Hence, the subsequent comparison to dipyridamole-induced coronary flow velocity response has been, pathophysiologically, invalid, because the prior PCI had variably influenced the haemodynamic stenosis relevance. In other words, the coronary artery stenosis of interest has to be left structurally unaltered in order to undergo functional severity testing by reactive hyperaemia. This can be safely performed by proximal diagnostic coronary balloon occlusion8.
In the present study, reactive hyperaemia was applied very systematically as a proximal coronary balloon occlusion of exactly one-minute duration (directly stenosis-adjacent distal occlusion in the few cases with ostial stenosis), immediately followed by a contrast bolus injection of a defined volume and volume flow rate. Thus, strictly speaking, reactive hyperaemia was the result of the ischaemia itself and the vasodilating effect of the coronary contrast injection. Since the latter was dosed up according to the left or right coronary artery as vessel of interest, variation in hyperaemic response was probably introduced in the context of varying myocardial masses supplied by the respective artery. However, the rationale of choosing coronary occlusion as a hyperaemic stimulus was less related to reducing variability in hyperaemic response, and more to augmenting its strength, and thus its potential to achieve maximal hyperaemia, which can only be reached if ischaemia is absolute. In this situation, i.e., in the absence of an alternative source of blood supply (collaterals) during coronary occlusion, it is a stronger hyperaemic stimulus than systemic adenosine. This was demonstrated by the present investigation, because FFR values were lower for a given stenosis using reactive hyperaemia than adenosine in case of a CFI less than 10% (Figure 6). Above this value, post-ischaemic FFR values were higher than adenosine-induced FFR due to the mitigating effect of functional collaterals on regional ischaemia. Hence, adenosine-induced FFR detected a structurally relevant stenosis more sensitively. Overall, adenosine-induced FFR was numerically more sensitive than reactive hyperaemia FFR in finding a relevant stenosis, but it was also less specific, and generally less accurate. Is there an explanation in addition to the stronger or weaker hyperaemic stimulus as induced by proximal coronary occlusion for the variable FFR response depending on collateral function?
REGIONAL VERSUS GLOBAL MYOCARDIAL HYPERAEMIA
The more sensitively detected stenosis by global hyperaemia in the presence of high collateral function is probably enhanced through the mechanism by which i.v. adenosine-induced hyperaemia manifests regional ischaemia, i.e., by coronary steal20. Coronary steal is a regional perfusion deficit during hyperaemia in favour of an adjacent territory, which itself is not or is less ischaemic. Steal is caused by regionally unbalanced microvascular resistance changes to coronary flow, being more substantial in the non-ischaemic than in the stenotic supply area. Steal is transmitted via arterial branches, inter-arterial anastomoses (collaterals) or both21, and it requires global myocardial hyperaemia as provoked by i.v. adenosine or by physical exercise (or alternatively, selective hyperaemia in the non-stenotic region). It has been previously documented that coronary steal occurs preferentially in the presence of well-functioning collaterals21. Thus, in patients with high CFI, the stimulus for global, hyperaemia-induced steal in the ischaemic area is greater than in those with poor collateral supply, thereby unmasking a haemodynamically relevant obstruction more sensitively. As a direct result, global, adenosine-induced hyperaemia FFR is less specific for stenosis detection and, overall, tends to be less accurate than regional reactive hyperaemia FFR.
Study limitations
The above-outlined differential effects of regional and global myocardial hyperaemia could have been further tested by evaluating an alternative regional hyperaemic stimulus, i.e., intracoronary adenosine. Obviously, this would have been only regional under the condition of the specific arterial stenosis distribution of an RCA stenosis with normal LCA. Vice versa, a “regional” intracoronary adenosine injection would have been “global” regarding the LCA. The present study was not powered to test clinical endpoints for the comparison between systemic adenosine-induced and regional reactive hyperaemia for FFR.
Conclusions
Regional reactive hyperaemia FFR is not inferior to systemic adenosine FFR in detecting structurally relevant coronary stenosis. Depending on the absence or presence of functional collaterals, systemic adenosine-induced FFR may underestimate or overestimate stenosis severity, respectively.
| Impact on daily practice The present study results imply that functional coronary stenosis severity can be obtained using post-occlusive reactive regional myocardial hyperaemia instead of global adenosine-induced FFR. The selection of percent diameter stenosis as the structural coronary reference parameter in this study does not imply that it ought to be preferred over a haemodynamic stenosis assessment in guiding the decision on PCI. |
Acknowledgements
We wish to thank Hélène Steck, RN, and Raphael Grossenbacher, RN, for their valuable work in patient recruitment and expert technical assistance in data acquisition.
Funding
This study was supported by grants from the Swiss National Science Foundation for research (grant #3200B_141030/1 to CS).
Conflict of interest statement
The authors have no conflicts of interest to declare.

